
Case Report
Chronic Dis Int. 2015;2(2): 1016.
Gilbert’s Syndrome and Proteinuria: A Case Report
Vaia D. Raikou1* and Despina Kyriaki2
1Department of Medicine, National & Kapodistrian University of Athens, Greece
2Department of Nuclear Medicine, General Hospital Athens, Greece
*Corresponding author: Vaia Raikou, Department of Medicine, National & Kapodistrian University of Athens, 17 Agiou Thoma -Athens, Greece
Received: May 13, 2015; Accepted: June 22, 2015; Published: June 23, 2015
Abstract
Gilbert’s syndrome is characterized by increased unconjugated bilirubin in the plasma. Bilirubin may be associated with chronic antihypertensive actions, due to its role on renal hemodynamic and renal tubule function. We describe a case of patient with Gilbert’s disease and proteinuria.
Keywords: Gilbert’s syndrome; Proteinuria; Bilirubin
Introduction
Gilbert’s syndrome, with a prevalence of 1% to 3% in general population, is a common, harmless genetic condition characterized by increased unconjugated bilirubin in the plasma, due to mutations in hepatic UDP-glucuronosyltransferase (UGT1A1), which decrease the conjugation of bilirubin in the liver. Bilirubin is derived from the breakdown of red blood cells produced heme by Heme Oxygenase (HO) enzymes in the spleen. In the blood, bilirubin travels to the liver and is conjugated by hepatic UGT1A1 enzymes. The conjugated bilirubin then exits the liver through the biliary ducts into the bile where it is eliminated through the digestive system.
The enzyme abnormality in Gilbert’s syndrome results in mild elevations of bilirubin in the blood, particularly after starvation or dehydration. The elevated bilirubin pigment can sometimes cause mild yellowing (jaundice) of the eyes. People with Gilbert syndrome are otherwise entirely normal with no other signs or symptoms. Their liver enzyme levels in blood serum are also entirely normal. Gilbert syndrome is most commonly diagnosed after puberty, when alterations in sex hormone levels cause the blood bilirubin levels to rise, or it is diagnosed in the course of routine blood screening. There is no need for treatment, and the prognosis is excellent.
Previous studies have shown that mutations in UGT1A1, such as in Gilbert’s syndrome, which resulted in moderated increases in plasma bilirubin (twofold) were protective against atherosclerosis, coronary heart disease, metabolic syndrome, diabetic nephropathy and end stage of renal disease [1,2,3]. Also, it has been reported that bilirubin is associated with chronic antihypertensive actions, due to its role on renal hemodynamic attenuating constriction from vasoconstrictors such as AII and also acting as an antioxidant in the body [4].
In present report, we describe a case of patient with Gilbert’s disease and proteinuria.
Case Description
A female Gilbert’s disease patient, 30 years old, is coming for examination of proteinuria after recommendation by a practitioner. Proteinuria was found in a annual checkup in urine spot since 2 years. The patient was an official, in a physical activity, with a Body Mass Index (BMI) equal to 28 and she was receiving food supplements.
Her past medical history was free of illnesses. The physical examination did not reveal any significant findings and she was receiving no drugs. She had a normal blood pressure. Her family history was positive for hypertension and dyslipidemia.
The laboratory measurements showed normal findings, except of a mild elevation of Gamma-glutamyltranspeptidase (γGT) and triglycerides. She had a fatty liver history. An urine spot examination showed a single proteinuria without hematuria nor cylindruria and with a normal specific gravity. The echocardiography examination of kidneys was normal and a triplex renal vascular examination was also normal.
Clearance creatinine was equal to 93 ml/min and capillary protein electrophoresis was normal (Figure 1). The average value of proteinuria of three sequences measurements in daily urine per 6 months was equal to 492.3 mg/24 hours. Urine protein electrophoresis and analysis with immunoreactions showed albumin fraction, a1-antitrypsin, transferrin and multiclonical bound with A, G, M immunoglobins κ chains excretion (Figure 2).
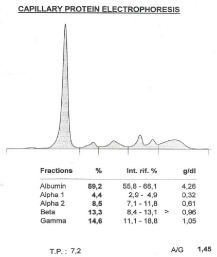
Figure 1: Capillary protein electrophoresis.
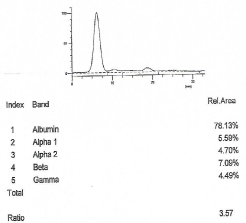
Figure 2: Urine protein electrophoresis.
We recommended follow up for daily proteinuria every 6 months, pause of food supplements and try for reduction of body weight to a normal BMI value.
Discussion
We report a case of patient in whom a mild hyperbilirubinemia due to Gilbert’s syndrome coexisted with single proteinuria less than 1 gr/day.
Previously, in a experimental model of moderate hyperbilirubinemia, the chronic infusion of angiotensin II (Ang II) did not result in a decrease in Glomerular Filtration Rate (GFR) [5]. The moderate hyperbilirubinemia may preserve the renal blood flow in glomerulus and normalize the renal vascular resistance in Ang II, affecting mainly the renal afferent arteriole hemodynamic. The regulation of GFR from renal afferent arteriole is achieved by both mechanisms, the myogenic response and the regulated by macula densa Tubuloglomerular Feedback (TGF).It has been already suggested that moderate increases in bilirubin protect against excessive TGF mediated vasoconstriction and can attenuate constriction to high levels of vasoconstrictors such as Ang II [6,7].
However, Ang II has a particular role on renal hemodynamic inducing more vasoconstriction on renal efferent arteriole than on renal afferent arteriole, resulting in preservation of GFR, even when the renal blood flow is decreased. The elevated constriction of renal efferent arteriole has as a result high filtration pressure in glomeruli, which protects against decreases of GFR in low blood volume conditions.
Additionally, the high filtration pressure in glomerulus causes increased translumenal pressure and proteinuria. This hypothesis may be an explanation for the found single proteinuria in our case report. A mild or moderate hyperbilirubinemia may have no the same Ang II mediated action on efferent than on afferent renal arteriole inside glomerulus, resulting in a single low proteinuria. In previous experimental models the role of a mild or moderate hyperbilirubinemia on the Ang II mediated vasoconstriction of renal efferent arteriole has not been investigated.
On the other hand, in pathophysiology, large proteins in the glomerular capillary such as albumin (molecular weight 55,000 Da) are almost completely held back from entering the filtrate in the glomerular capsule by the filtration barrier made up of the podocytes, the glomerular basement membrane and the endothelial cells of the glomerular capillaries. However, small proteins (< 4nm diameter) are freely filtered dependent on molecular size, conformation and charge. Whatever protein is filtered is almost completely reabsorbed in the proximal tubule, where it is catabolized by tubular cells. A small quantity of protein in the tubular fluid comes from another source, as secretion by the cells lining the tubules. The upper limit of normal for the total amount of protein excreted into the urine over a day is 150 to 200 mg and for albuminuria, 30 mg/day is taken as the upper limit of normal.
As the filtration barrier is destructed by pathologic processes, increasing amounts of protein leak out into the glomerular filtrate, overwhelming the capacity of the tubules to absorb and metabolize the leaked protein having as a result proteinuria. In disease, the amount of proteinuria may increase dramatically and proteinuria > 1gr/day is typically due to glomerular damage. However, lower level is caused by tubular damage, leading to failure of reabsorption of filtered proteins, rather than by glomerular injury. Indeed, in our reported patient the low proteinuria (< 1gr/day) without hematuria nor cylindruria, including albumin fraction, a1-antitripsin, transferrin and multiclonical light k chains, in combination to normal capillary protein electrophoresis and normal kidney function could due to renal tubular failure of reabsorption and metabolism them, rather than to pathological protein overproduction, neither increased filtration them by glomerular injury.
Based on the above, the proteinuria less than 1gr/day of our reported Gilbert’s syndrome patient could be explained by a toxic impact of mild chronic bilirubinemia on renal tubules resulting in a failure of metabolism of filtered proteins, in combination to a possible role of bilirubinemia on hemodynamic function of efferent renal arteriole and not only on the function of afferent renal arteriole, as previously has been reported. However, the proteinuria may be independent on bilirubinemia and our patient may have an inadequacy of renal tubular cells for reabsorption and catabolism of filtered proteins, irrelevant to bilirubinemia. This tubular inadequacy may be connected to receiving food supplements by our patient.
The quantity of proteinuria is correlated with the risk of progressive decline in kidney function, although this relationship varies in specific diseases. Whether this simply reflects severity of the glomerular injury, or if the risk is caused by the direct toxicity of proteins to the tubule remains controversial. Proteinuria is also associated with an increased risk of cardiovascular events [8]. For these reasons, proteinuria is an indication for kidney biopsy, commonly total proteinuria > 1gr/day, or > 450mg/day, if it is associated with hematuria. Also, proteinuria > 0.5-1gr/day may be used as an indication for treatment with angiotensin-converting enzyme inhibitors.
Conclusion
In Gilbert’s syndrome patients with mild bilirubinemia may coexist single low proteinuria. These patients must be in watch-andwait for daily proteinuria once a year or frequently, may be made renal biopsy and start treatment for reduction of proteinuria.
References
- Novotny L, Vitek L. Inverse relationship between serum bilirubin and atherosclerosis in men: a meta-analysis of published studies. Exp Biol Med (Maywood). 2003: 228: 568-571.
- Han SS, Na KY, Chae DW, kim YS, Kim S, Chin HJ, et al. High serum bilirubin is associated with the reduced risk of diabetes mellitus and diabetic nephropathy. J Exp Med. 2010: 221: 133-140.
- Wu Y, Li M, Xu M, Bi Y, Li X, Chen Y, et al. Low serum total bilirubin concentrations are associated with increased prevalence of metabolic syndrome in Chinese. J Diabetes. 2011: 3: 217-224.
- David E. Stec, Peter A. Hosick , Joey P. Granger. Bilirubin, renal hemodynamics and blood pressure. Front Pharmacol. 2012; 3: 18.
- Vera T, Stec DE. Moderate hyperbilirubinemia improves renal hemodynamics in ANGII- dependent hypertension. Am J Physiol Regul Integr Comp Physiol. 2010; 299: R1044-R1049.
- Ren Y, D’Ambrosio MA, Wang H, Liu R, Garvin JL, Carretero OA. Hemeoxygenase metabolites inhibit tubuloglomerular feedback (TGF). Am J Physiol Renal Physiol. 2008: 295: F1207-F1212.
- Wang H, Garvin JL, D’Ambrosio MA, Falck JR, Leung P, Liu R, et al. Heme oxygenase metabolites inhibit tubuloglomerular feedback in vivo. Am J Physiol Heart Circ Physiol. 2011; 300: H1320-H1326.
- Wang H, Garvin JL, D’Ambrosio MA, Falck JR, Leung P, Liu R, et al. Heme oxygenase metabolites inhibit tubuloglomerular feedback in vivo. Am J Physiol Heart Circ Physiol. 2011; 300: H1320-H1326.
- Matsushita K, van der Velde M, Astor BC, Woodward M, Levey AS, de Jong PE, et al. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet. 2010; 375: 2073-2081.