Review Article
Austin J Clin Cardiolog. 2014;1(3): 1019.
Optical Coherence Tomography in Coronary Artery Disease: Toward Sub cellular Imaging
Manabu Kashiwagi1, Linbo Liu2, Joseph A. Gardecki1 and Guillermo J. Tearney1,3,4*
1Harvard Medical School and Wellman Center for Photo medicine, Massachusetts General Hospital, USA
2School of Electrical & Electronic Engineering and School of Chemical & Biomedical Engineering, Nanyang Technological University, Singapore
3Harvard-MIT, Division of Health Sciences and Technology, USA
4Department of Pathology, Harvard Medical School and Massachusetts General Hospital, USA
*Corresponding author: Guillermo J. Tearney, Department of Pathology, Harvard Medical School and Massachusetts General Hospital, 55 Fruit Street, BHX604A, Boston MA 02114
Received: March 24, 2014; Accepted: April 21, 2014; Published: April 24, 2014.
Abstract
Intracoronary optical coherence tomography (OCT) is an interferometric imaging technology that uses near–infrared light to provide cross–sectional images with an axial resolution of 10 µm and a transverse of 20–40 µm in vivo. The imaging capabilities of OCT have enabled visualization of important features of coronary plaque, including thrombus, macrophage, neovascularization, stent implantation and stent strut coverage, which have provided new insights for better understanding of this disease. Frequency domain (FD)–OCT is secondgeneration form of OCT that is able to acquire OCT images at a much higher frame. The high–speed imaging capabilities of FD–OCT have made intravascular OCT practical and the introduction of this new technology is expected to help cardiologists make more informed decisions on coronary interventions. Recently, a new form of OCT, termed micro–optical coherence tomography (µOCT), has been developed, which affords a ten–times spatial resolution improvement compared conventional OCT systems. µOCT has shown to be capable of imaging sub cellular features of coronary artery that are relevant to atherosclerosis, including leukocyte adhesion and diapedesis, fibrin and platelet accumulation, and individual macrophages, smooth muscle cells, and cholesterol crystals. In addition, µOCT is capable of evaluating stent struts and the body’s reaction to implantable devices in much greater detail than previously possible. These unique capabilities of µOCT could make it a useful tool for understanding and diagnosing coronary artery disease at the cellular level.
Keywords: Optical Coherence Tomography; Atherosclerosis; Coronary Artery
Introduction
Optical coherence tomography (OCT) is an emerging optical imaging modality that performs high–resolution cross–sectional imaging of tissue microstructure in situ in real–time [1]. When applied to cardiology, OCT can visualize coronary pathology with a transverse resolution of 20–40 µm and an axial resolution of ˜10 µm, which are one to two orders of magnitude better than intravascular ultrasound (IVUS). Similar to IVUS, OCT measures time–of–flight of back–reflected radiation from the coronary wall to construct a depthresolved reflectivity profile. OCT images are higher in resolution than IVUS because the propagation of light is faster than sound. Scanning the beam along the arterial wall generates a three–dimensional volumetric data set that captures comprehensive micro structural information of the coronary artery.
There are two implementations of OCT referred to as timedomain (TD)–OCT and frequency–domain (FD)–OCT [2–5]. In TDOCT system, light from a broadband light source (around 1300 nm wavelength) is split into a sample arm that is focused on the coronary wall and a reference arm that is sent to a moving reference mirror. The back–reflected light from the sample and reference arms are recombined at a single detector. When the optical path difference between light reflected from various structures in the coronary wall (sample arm) and the reference arm are within coherence parameter of the light source termed its coherence length, the light from these two arms combine and form an interference pattern. Depth–resolved arterial structure is constructed by recording the interferometric intensity as a function of reference mirror distance as it is moved. An OCT image is generated by successively moving the focus to a new location on the coronary wall and performing a TD–OCT scan.
Many of the seminal studies that established OCT as an intracoronary diagnostic imaging technology were conducted with TD–OCT. Using cadaver specimens, TD–OCT ex vivo studies demonstrated OCT’s ability to visualize the three–layered structure of a coronary artery and established criteria for diagnosing coronary atherosclerosis [6]. Post mortem studies revealed that OCT could distinguish different types of coronary artery plaques with high sensitivity and specificity [7]. Furthermore, a number of important hallmarks of the disease such as macrophage accumulations, thrombus classification, cholesterol crystals, calcium deposition, fibrous caps, and lipid cores could be visualized [8–11].
Because of OCT’s superior contrast and resolution, this technology is able to assess coronary stents following placement. In a swine study conducted in 2000, OCT was able to more accurately detect artery dissection, tissue prolapsed and malapposition than IVUS [12]. The first–in–human OCT imaging case was reported in 2002 [6] with a number of clinical pilot studies [13–16]. From these in vivo OCT studies, we have gained a more detailed understanding of human atherosclerotic plaques, thrombus formation, macrophage distributions, neovascularization, stent implantation and stent strut coverage in coronary artery disease [13–21]. In particular, OCT clinical trials have contributed to understanding the morphological features associated with acute coronary syndrome (ACS), caused by plaque rupture and thrombus formation. OCT is a unique intracoronary imaging technology that has demonstrated the ability to quantify the thickness of a thin fibrous cap and to detect the presence of a large lipid pool and macrophage infiltration within fibrous cap, all of the pathological hallmarks of high–risk or vulnerable plaques that are thought to be precursors of ACS and AMI [22]. In addition, in–vivo OCT studies have shown the capability of this technology to study key factors presumably related to the prognosis of stent–implanted lesions, including stent strut coverage, neointimal hyperplasia, stent mal apposition [23,24] and in–stent neoatherosclerosis [25,26].
Widespread clinical adoption of TD–OCT was limited due to slow acquisition speed, typically 4–8 frames per second. As a result, most TD–OCT imaging studies required balloon occlusion of the coronary with flushing to remove blood from the field of view. This balloon occlusion technique limited the applicability of IVOCT because of long scan times with a potential for inducing coronary damage or myocardial ischemia.
A major technical breakthrough in IVOCT occurred with the development of Fourier–Domain (FD)–OCT, a second–generation form of OCT that is able to obtain OCT images with a much higher frame rate compared with TD–OCT, while maintaining excellent image quality. There are two types of FD–OCT; one that utilizes a wavelength swept laser source, often referred to as SS–OCT or optical frequency domain imaging (OFDI), and another that utilizes a broad bandwidth optical source termed spectral–domain systems (SD–OCT).In SS–OCT or OFDI systems, the reference mirror is fixed so that the reference and sample arm path lengths are roughly equivalent. The light source has a narrow instantaneous line width but its wavelength is rapidly swept over a broadband spectral range. Each wavelength component is undergoes interference between the back–reflected light of the sample and reference arm. Applying a Fourier transform to the spectral interference pattern yields an OCT reflectively profile and the depth–resolved coronary structure. Because the swept source laser can be tuned more rapidly than the reference mirror can be moved in TD–OCT, the OFDI frame rate is greater than that of TDOCT by 1–2 orders of magnitude, which enables the acquisition of three–dimensional comprehensive volumetric microscopy of long arterial segment following a safely administered single 8–10 cc saline or radio contrast flush.
The initial demonstration of intracoronary OFDI in–vivo was performed in a swine in 2006 [5]. After FDA approval of the OFDI catheter, the first clinical study was conducted in 2008 [27]. Following this initial demonstration, IVOCT products based on this technology have been commercialized and are widely available for use ininterventional cardiology. The near term goal for this technology is to improve outcomes for coronary intervention and follow the response of stent deployment [28,29]. To date, adoption of OFDI by leading cardiovascular centers is growing due to the improvements in the technology and efforts to standardize technology among different cardiovascular research centers and manufacturers [30].
Although OCT provides greater than an order of magnitude increase in resolution over IVUS, higher–resolution images are required to further explore cellular–level responses to coronary atherosclerosis. We have recently demonstrated a new higherresolution form of OCT, termed micro–optical coherence tomography (µOCT), which affords ten–time better spatial resolution than that of conventional FD–OCT systems [31]. Based on a form of spectraldomain(SD)–OCT, µOCT differs from OFDI in that it uses a larger spectral bandwidth and shaped optical beam that illuminates theartery wall, which produces high resolution images in both axial (≤1 µm) and lateral (≤2 µm) directions. With this improved resolution, µOCT has demonstrated the ability to visualize subcellular features of coronary artery relevant to atherosclerosis ex vivo, including leukocyte adhesion and diapedesis, fibrin and platelet accumulation,macrophage, smooth muscle cells, and cholesterol crystals (Figure 1). In addition, µOCT has been shown ex vivo to be capable of evaluating polymer coating overlying stent struts (Figure 2) as well as inflammatory cell infiltrations around these devices. Current µOCTresearch is focused on the development of a catheter that is capable of acquiring this information in vivo. Once intravascular µOCT is available, it is likely that the cellular and subcellular resolution of thistechnology will provide significant insight into the nature of human coronary artery disease, will provide superior diagnostic capabilities for the prospective prevention of ACS and AMI, and will enable the design and understanding of the arterial response to implanted coronary medical devices.
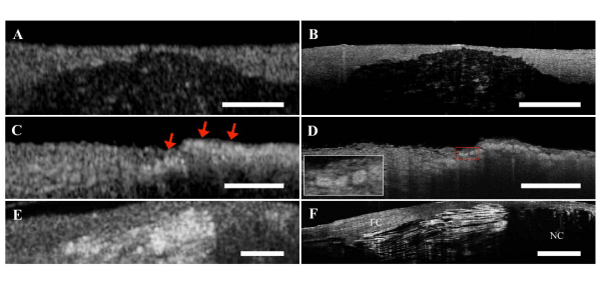
(A,B) Comparison between corresponding FD–OCT and µOCT images of a calcium plate within coronary artery wall. (C) Foam cells imaged by FDOCT appear as highly scattering, ill–defined punctate regions (red arrows). (D) Corresponding µOCT image of foam cells. µOCT clearly visualizes each foam cell individually, which appear as highly scattering round or ellipsoidal structures (Left lower inset) that contain smaller low signal regions within, consistent with nuclei. (E) Necrotic fibroatheroma with cholesterol crystal by FD–OCT. (F) Cholesterol crystals are characterized by intense reflections from their top and bottom surfaces on µOCT. Scale bar, 200 µm. FC; Fibrous Cap, NC; Necrotic Core.
Figure 1: Corresponding µOCT and FD–OCT images of human coronary artery ex vivo.(A,B) Comparison between corresponding FD–OCT and µOCT images of a calcium plate within coronary artery wall. (C) Foam cells imaged by FDOCT appear as highly scattering, ill–defined punctate regions (red arrows). (D) Corresponding µOCT image of foam cells. µOCT clearly visualizes each foam cell individually, which appear as highly scattering round or ellipsoidal structures (Left lower inset) that contain smaller low signal regions within, consistent with nuclei. (E) Necrotic fibroatheroma with cholesterol crystal by FD–OCT. (F) Cholesterol crystals are characterized by intense reflections from their top and bottom surfaces on µOCT. Scale bar, 200 µm. FC; Fibrous Cap, NC; Necrotic Core.
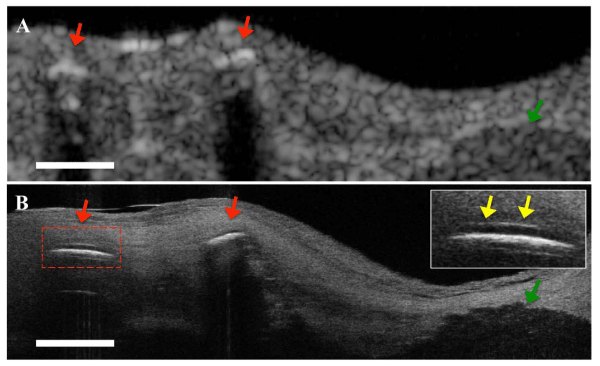
(A) OFDI image. Stent struts (red arrows) and calcified lesion (green arrow) can be seen. (B) Corresponding µOCT image. Drug eluting stents showing polymer (yellow arrows) overlying the strut reflections (top right inset) and calcified lesions were observed. Scale bar, 200 µm.
Figure 2: Comparison µOCT and FD–OCT images of stent implanted coronary lesion ex vivo.(A) OFDI image. Stent struts (red arrows) and calcified lesion (green arrow) can be seen. (B) Corresponding µOCT image. Drug eluting stents showing polymer (yellow arrows) overlying the strut reflections (top right inset) and calcified lesions were observed. Scale bar, 200 µm.
Acknowledgement
Sources of funding
This review is based in part on research that was supported by NIH grants R01HL093717 and R01HL076398.
Disclosure statement
Dr. Tearney receives catheter materials from Terumo Corporation. Massachusetts General Hospital has a licensing arrangement with Terumo Corporation. Dr. Tearney has the rights to receive royalties from this licensing arrangement. Dr. Tearney receives sponsored research funding from Canon Inc. Dr. Tearney consults for Samsung Advanced Institute of Technology. Dr. Tearney receives sponsored research from Merck.
References
- Huang D, Swanson EA, Lin CP, Schuman JS, Stinson WG, Chang W, et al. Optical coherence tomography. Science. 1991; 254: 1178-1781.
- Tearney GJ, Brezinski ME, Bouma BE, Boppart SA, Pitris C, Southern JF, et al. In vivo endoscopic optical biopsy with optical coherence tomography. Science. 1997; 276: 2037-2039.
- Leitgeb R, Hitzenberger C, Fercher A. Performance of fourier domain vs. time domain optical coherence tomography. Opt Express. 2003; 11: 889-894.
- Choma M, Sarunic M, Yang C, Izatt J. Sensitivity advantage of swept source and Fourier domain optical coherence tomography. Opt Express. 2003; 11: 2183-2189.
- Yun SH, Tearney GJ, Vakoc BJ, Shishkov M, Oh WY, Desjardins AE, et al. Comprehensive volumetric optical microscopy in vivo. Nat Med. 2006; 12: 1429-1433.
- Jang IK, Bouma BE, Kang DH, Park SJ, Park SW, Seung KB, et al. Visualization of coronary atherosclerotic plaques in patients using optical coherence tomography: comparison with intravascular ultrasound. J Am Coll Cardiol. 2002; 39: 604-609.
- Yabushita H, Bouma BE, Houser SL, Aretz HT, Jang IK, Schlendorf KH, et al. Characterization of human atherosclerosis by optical coherence tomography. Circulation. 2002; 106: 1640-1645.
- Tearney GJ, Yabushita H, Houser SL, Aretz HT, Jang IK, Schlendorf KH, et al. Quantification of macrophage content in atherosclerotic plaques by optical coherence tomography. Circulation. 2003; 107: 113-119.
- Kume T, Akasaka T, Kawamoto T, Ogasawara Y, Watanabe N, Toyota E, et al. Assessment of coronary arterial thrombus by optical coherence tomography. Am J Cardiol. 2006; 97: 1713-1717.
- Tearney GJ, Jang IK, Bouma BE. Evidence of Cholesterol Crystals in Atherosclerotic Plaque by Optical Coherence Tomographic (OCT) Imaging. Eur. Heart J. 2003: 23; 1462.
- Tearney GJ, Yabushita H, Houser SL, Aretz HT, Jang IK, Schlendorf KH, et al. Quantification of macrophage content in atherosclerotic plaques by optical coherence tomography. Circulation. 2003; 107: 113-119.
- Tearney GJ, Jang IK, Kang DH, Aretz HT, Houser SL, Brady TJ, et al. Porcine coronary imaging in vivo by optical coherence tomography. Acta Cardiol. 2000; 55: 233-237.
- Jang IK, Tearney GJ, MacNeill B, Takano M, Moselewski F, Iftima N, et al. In vivo characterization of coronary atherosclerotic plaque by use of optical coherence tomography. Circulation. 2005; 111: 1551-1555.
- MacNeill BD, Jang IK, Bouma BE, Iftimia N, Takano M, Yabushita H, et al. Focal and multi-focal plaque macrophage distributions in patients with acute and stable presentations of coronary artery disease. J Am Coll Cardiol. 2004; 44: 972-979.
- Tanaka A, Imanishi T, Kitabata H, Kubo T, Takarada S, Kataiwa H, et al. Distribution and frequency of thin-capped fibroatheromas and ruptured plaques in the entire culprit coronary artery in patients with acute coronary syndrome as determined by optical coherence tomography. Am J Cardiol. 2008; 102: 975-979.
- Raffel OC, Tearney GJ, Gauthier DD, Halpern EF, Bouma BE, Jang IK, et al. Relationship between a systemic inflammatory marker, plaque inflammation, and plaque characteristics determined by intravascular optical coherence tomography. Arterioscler Thromb Vasc Biol. 2007; 27: 1820-1827.
- Raffel OC, Merchant FM, Tearney GJ, Chia S, Gauthier DD, Pomerantsev E, et al. In vivo association between positive coronary artery remodelling and coronary plaque characteristics assessed by intravascular optical coherence tomography. Eur Heart J. 2008; 29:1721-1728.
- Kitabata H, Tanaka A, Kubo T, Takarada S, Kashiwagi M, Tsujioka H, et al. Relation of microchannel structure identified by optical coherence tomography to plaque vulnerability in patients with coronary artery disease. Am J Cardiol. 2010; 105: 1673-1678.
- Sawada T, Shite J, Shinke T, Watanabe S, Otake H, Matsumoto D, et al. Persistent malapposition after implantation of sirolimus-eluting stent into intramural coronary hematoma: optical coherence tomography observations. Circ J. 2006; 70: 1515-1519.
- Matsumoto D, Shite J, Shinke T, Otake H, Tanino Y, Ogasawara D, et al. Neointimal coverage of sirolimus-eluting stents at 6-month follow-up: evaluated by optical coherence tomography. Eur Heart J. 2007; 28: 961-967.
- Toutouzas K, Karanasos A, Tsiamis E, Riga M, Drakopoulou M, Synetos A, et al. New insights by optical coherence tomography into the differences and similarities of culprit ruptured plaque morphology in non-ST-elevation myocardial infarction and ST-elevation myocardial infarction. Am Heart J. 2011; 161: 1192-1199.
- Virmani R, Burke AP, Farb A, Kolodgie FD. Pathology of the vulnerable plaque. J Am Coll Cardiol. 2006; 47: C13-18.
- Guagliumi G, Costa MA, Sirbu V, Musumeci G, Bezerra HG, Suzuki N, et al. Strut coverage and late malapposition with paclitaxel-eluting stents compared with bare metal stents in acute myocardial infarction: optical coherence tomography substudy of the Harmonizing Outcomes with Revascularization and Stents in Acute Myocardial Infarction (HORIZONS-AMI) Trial. Circulation. 2011; 123: 274-281.
- Guagliumi G, Musumeci G, Sirbu V, Bezerra HG, Suzuki N, Fiocca L, et al. Optical coherence tomography assessment of in vivo vascular response after implantation of overlapping bare-metal and drug-eluting stents. JACC Cardiovasc Interv. 2010; 3: 531-539.
- Kang SJ, Mintz GS, Akasaka T, Park DW, Lee JY, Kim WJ, et al. Optical coherence tomographic analysis of in-stent neoatherosclerosis after drug-eluting stent implantation. Circulation. 2011; 123: 2954-2963.
- Takano M, Yamamoto M, Inami S, Murakami D, Ohba T, Seino Y, et al. Appearance of lipid-laden intima and neovascularization after implantation of bare-metal stents extended late-phase observation by intracoronary optical coherence tomography. J Am Coll Cardiol. 2009; 55: 26-32.
- Tearney GJ, Waxman S, Shishkov M, Vakoc BJ, Suter MJ, Freilich MI, et al. Three-dimensional coronary artery microscopy by intracoronary optical frequency domain imaging. JACC Cardiovasc Imaging. 2008; 1: 752-761.
- Imola F, Mallus MT, Ramazzotti V, Manzoli A, Pappalardo A, Di Giorgio A, et al. Safety and feasibility of frequency domain optical coherence tomography to guide decision making in percutaneous coronary intervention. EuroIntervention 2010; 6: 575-581.
- Prati F, Di Vito L, Biondi-Zoccai G, Occhipinti M, La Manna A, Tamburino C, et al. Angiography alone versus angiography plus optical coherence tomography to guide decision-making during percutaneous coronary intervention: the Centro per la Lotta contro l'Infarto-Optimisation of Percutaneous Coronary Intervention (CLI-OPCI) study. EuroIntervention. 2012; 8: 823-829.
- Tearney GJ, Regar E, Akasaka T, Adriaenssens T, Barlis P, Bezerra HG, et al; Consensus standards for acquisition, measurement, and reporting of intravascular optical coherence tomography studies: a report from the International Working Group for Intravascular Optical Coherence Tomography Standardization and Validation. J Am Coll Cardiol 2012; 59: 1058-1072.
- Liu L, Gardecki JA, Nadkarni SK, Toussaint JD, Yagi Y, Bouma BE, et al. Imaging the subcellular structure of human coronary atherosclerosis using micro-optical coherence tomography. Nat Med. 2011; 17: 1010-1014.