
Case Report
Austin J Clin Cardiolog. 2016; 3(2): 1047.
Catheter Ablation for Ventricular Premature Contraction Triggering Electrical Storm
Lee W-C, Chen H-C, Chen Y-L and Chen M-C*
Division of Cardiology, Kaohsiung Chang Gung Memorial Hospital, Chang Gung University College of Medicine, Taiwan
*Corresponding author: Mien-Cheng Chen, Division of Cardiology, Department of Internal Medicine, Kaohsiung Chang Gung Memorial Hospital, Chang Gung University College of Medicine, 123 Ta Pei Road, Niao Sung District, Kaohsiung City, 83301, Taiwan
Received: July 16, 2016; Accepted: August 04, 2016; Published: August 05, 2016
Abstract
Electrical storm, defined as =3 episodes of Ventricular Tachycardia (VT) or Ventricular Fibrillation (VF) occurring within 24 hours, is a life-threatening medical emergency. A 73-year-old gentleman, with a history of diabetes mellitus and hypertension, presented with congestive heart failure. An Electrocardiography (ECG) showed borderline ST-T changes over anterior wall and high sequential blood troponin-I levels were noted. Accordingly, non-ST elevation myocardial infarction, Killip III, was diagnosed. Percutaneous coronary intervention was performed with one drug-eluting stent placement into middle left anterior descending artery and two drug-eluting stents placement into proximal-tomiddle right coronary artery under Intra-Aortic Balloon Pumping (IABP) support. Three days later, he experienced electrical storm in spite of amiodarone and lidocaine administration and deep sedation. Extracorporeal Membrane Oxygenation (ECMO) setup was inserted for electrical storm. Bedside lead II ECG showed on ventricular premature beat triggering VF. Therefore, he received radiofrequency catheter ablation under ECMO, IABP and ventilator support. 3D mapping by Ensite Navix mapping system demonstrated border zone along the left posterior fasicle and large scar area distributed across the inferior and anteroseptal wall of left ventricle. Purkinje-like potentials were registered at the border zone. Radiofrequency energy was appiled along the border zone from the inferoposteroseptal to anteroseptal wall of left ventricle till elimination of Purkinje potentials and substrate modification of the border zone. No more VT/VF could be induced by programmed ventricular stimulation after ablation. Subsequently, an Implantable Cardioverter Defibrillator (ICD) was implanted and ICD interrogation did not show any episode of VF/VF during the subsequent 3-month follow-up period.
Keywords: Catheter ablation; Electrical storm; Ventricular fibrillation; Ventricular premature contraction
Introduction
Electrical storm due to Ventricular Tachycardia/Ventricular Fibrillation (VT/VF) is a life-threatening medical emergency and a challenging problem for physicians. Refractory electrical storm can happen even through intensive antiarrhythmic administration, deep sedation and mechanical support. We describe here a case of catheter ablation for ventricular premature contraction triggered VF even under Extracorporeal Membrane Oxygenation (ECMO) and Intra- Aortic Balloon Pumping (IABP) support.
Case Report
A 73-year-old gentleman experienced shortness of breath gradually to orthopnea and bilateral legs edema in recent two months. He had medical history of type 2 diabetes mellitus and hypertension. Electrocardiography (ECG) showed sinus tachycardia, right bundle branch block, and borderline ST-T changes in the leads of anterior wall. High sequential blood troponin-I levels were noted. Non-ST segment elevation myocardial infarction, Killip III, was diagnosed. Chest radiography showed cardiomegaly and pulmonary congestion. IABP was inserted for hemodynamic support before percutaneous coronary intervention. Coronary angiography showed two-vessel coronary artery disease as Left Anterior Descending Artery (LAD) and Right Coronary Artery (RCA) totally occluded (Figure 1A, 1B). Percutaneous coronary intervention was performed with one drug-eluting stent placement into middle-LADafter thrombectomy and two drug-eluting stents placement into proximal- to middle- RCA under intravascular ultrasound guidance. Final coronary angiography showed Thrombolysis In Myocardial Infarction 3 flow in targeted vessels (Figure 1C, 1D). Three days later, ventilator and IABP were removed due to fair hemodynamic condition. Subsequently, he experienced shortness of breath and frequent ventricular tachyarrhythmias. After resuscitation, intubation was reinitiated and IABP was inserted again for frequent unstable ventricular tachyarrhythmias. Bedside lead II ECG monitor showed one Ventricular Premature Contraction (VPC) triggered VF (Figure 2). VF recurred for more than twenty times, in spite of amiodarone and lidocaine administration and fully sedation. ECMO setup was inserted for electrical storm. Repeat coronary angiography showed no significant stenosis over previous stented segments and one bare-metal stent was placed into a borderline lesion of ramus branch. After total revascularization, several episodes of ventricular tachyarrhythmia still recurred after weaning him off lidocaine administration. Therefore, he received radiofrequency catheter ablation for electrical storm.
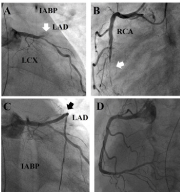
Figure 1: Coronary angiography. (A) Left Anterior Descending Artery (LAD)
totally occluded (white arrow), (B) Right Coronary Artery (RCA) totally
occluded (white arrow), (C) fair flow of LAD after stenting, (D) fair flow of RCA
after stenting.
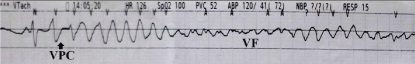
Figure 2: Bedside rhythm strip in lead II. One ventricular premature
contraction triggered Ventricular Fibrillation (VF).
Catheter ablation was performed under ECMO, IABP and ventilator support (Figure 3A). Clinical VPC showed left axis deviation and right bundle branch block, implicating left ventricular origin. An 8 French arterial sheath was placed above the IABP sheath. Left ventriculogram presented diffuse hypokinesia and poor left ventricular performance with an ejection fraction of 25%. Unfortunately, complete atrioventricular block developed after left ventriculography. A 4 Frenchdecapolar mapping catheter was introduced percutaneously into the right femoral vein through a 5 French venous sheath, which was positioned in the right ventricular apex across the tricuspid valve. IABP function was hold during mapping and ablation. Left ventricular geometry and voltage mapping (setting 0.2-0.8 mV) were created with a 7 Frenchquadripolar irrigation ablation catheter and Ensite Navix 3D mapping system (St Jude, St Paul, MN, USA) (Figure 3A). The posterior view of voltage map showed border zone along the left posterior fasicle (Figure 3B). The right anterior oblique view of voltage map showed large scar area distributed acrossthe inferior and anteroseptal wall of left ventricle (Figure 3C). Purkinje-like potentials were registered at the border zone by ablation catheter (Figure 3D). VF (Figure 3E) and VT (Figure 3F) were triggered during ablation at the border zone with Purkinjelike potentials. The morphology of triggered VT was similar to clinical VPC. Pace-mapping at the ablation site demonstrated 12/12 perfect match. Radiofrequency energy 50 Watts (target of 48 Celcius) for 30 seconds at each point was appiled along the border zone from the inferoposteroseptal to anteroseptal wall of left ventricle. Substrate modification of the border zone by ablation was performed till elimination of Purkinje potentials and local voltage less than 0.1 mV were achieved at the border zone. The total ablation area was 6.1 cm2 (Figure 3G). No more VT/VF could be induced by programmed right ventricular stimulation up to triple extra stimuli after ablation. Two days later, an Implantable Cardioverter Defibrillator (ICD) was implanted. IABP was removed one day later, ECMO was removed three days later, and ventilator support was removed one month later. ICD interrogation did not show any episode of VF/VFduring the subsequent 3-month follow-up period.

Figure 3a: (A) Catheter ablation was performed using Ensite Navix 3D mapping
system (St Jude, St Paul, MN, USA) in the presence of extracorporeal
membrane oxygenation and intra-aortic balloon pumping support.
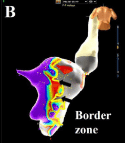
Figure 3b: (B) The posterior view of voltage map showed border zone along
the left posterior fascicle (black arrows) (setting 0.2-0.8 mV).
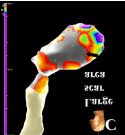
Figure 3c: (C) The right anterior oblique view of voltage map showed large
scar area distributed at the inferior and anteroseptal wall of left ventricle
(black arrow).
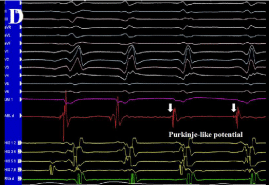
Figure 3d: (D) Purkinje-like potential at border zone (white arrows).
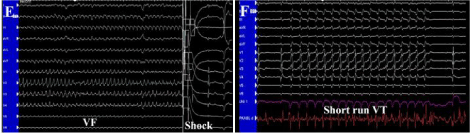
Figure 3 e& f: (E, F) Short-run ventricular tachycardia (VT) and ventricular fibrillation (VF) were triggered during ablation over left posterior fascicular site.
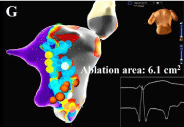
Figure 3g: (G) The total ablation area was 6.1 cm2 (blue points) with elimination
of Purkinje potentials and substrate modification of the border zone.
Discussion
Electrical storm is a life-threatening medical emergency that is defined as =3 episodes of VT or VF occurring within 24 hours and requiring intervention [1]. The mechanisms of electrical storm include enhanced sympathetic tone, ischemia, electrolyte imbalances, genetic abnormalities (such as Brugada syndrome, long QT syndrome, short QT syndrome, arrhythmogenic right ventricular dysplasia), iatrogenic (ICD related), or endocrine disorders (thyroid disorders, pheochromocytoma) [2]. If VT leads to hemodynamic instability or if substantial comorbidities are present, admission to an intensive care unit is mandatory, and in addition to antiarrhythmics, sedation, intubation, as well as mechanical hemodynamic support have to be considered [3]. Electrical storm carried a poor prognosis and increased 3.15-fold risk of all-cause mortality [4].
Urgent catheter ablation is recommended as class IB indication for scar-related incessant VT or electrical storm [5]. Several studies have proven the efficacy of catheter ablation for electrical storm [6,7]. VF can be initiated by VPCs during the vulnerable period of cardiac repolarization. Targets for VF triggers include VPCs preceded by Purkinje potentials and VPCs from the right ventricular outflow tract in structurally normal hearts, as well as VPCs preceded by Purkinje potentials in ischemic cardiomyopathy [8]. The earliest activation site of VPCs with Purkinje activation should be sought and ablated. However, substrate modification of the Purkinje network may be applied when the earliest site cannot be determined or is located close to the atrioventricular node [8]. Dissociated firing from the Purkinje network is sometimes observed after a successful suppression of VF by catheter ablation with substrate modification of Purkinje network [8]. In addition, previous study showed that catheter ablation of VT can effectively reduce the number of VT recurrences [9]. Therefore, catheter ablation of VT is very important for the prevention of ICD interventions in the patients with ICD [10].
In our case, this patient experienced refractory electrical storm, despite antiarrhythmics administration, deep sedation and mechanical supports. Catheter ablation was performed along the border zone of scar with elimination of Purkinje-like potentials and substrate modification. The voltage definition of the scar is critical for identifying isthmus channels in myocardial infarctions, and the majority of conducting channels are identified when the scar voltage was set at = 0.2 mV [11]. We set voltage zone between 0.2 and 0.8 mV to detect the area of scar and prevent ablating large area. Another challenge of this case was the difficulty to achieve femoral vascular access for ablation and mapping in the presence of ECMO and IABP placement. Here, we report a complicated case of electrical storm managed by catheter ablation.
Conclusion
Catheter ablation is an effective and feasible method to deal with electrical storm in patients with ischemic cardiomyopathy, even in the presence of ECMO and IABP placement.
References
- Zipes DP, Camm AJ, Borggrefe M, Buxton AE, Chaitman B, Fromer M et al. ACC/AHA/ESC 2006 guidelines for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: a report of the American College of Cardiology/American Heart Association Task Force and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Develop Guidelines for Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death). European Heart Rhythm Association; Heart Rhythm Society. J Am CollCardiol. 2006; 48: 247-346.
- JC Hsieh, Michael Bui, Srinivas Yallapragda, Shoei K, Stephen Huang. Current Management of Electrical Storm. ActaCardiol Sin. 2011; 27: 71-76.
- Haegeli LM, Della Bella P, Brunckhorst CB. Management of a Patient With Electrical Storm: Role of Epicardial Catheter Ablation. Circulation. 2016; 133: 672-676.
- Federico Guerra, Matilda Shkoza, Lorena Scappini, Marco Flori, Alessandro Capucci. Role of electrical storm as a mortality and morbidity risk factor and its clinical predictors: a meta-analysis. Europace. 2014; 16: 347-353.
- Priori SG, Blomström-Lundqvist C, Mazzanti A, Blom N, Borggrefe M, Camm J. 2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: The Task Force for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death of the European Society of Cardiology (ESC). Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC). Eur Heart J. 2015; 36: 2793-2867.
- Carbucicchio C, Santamaria M, Trevisi N, Maccabelli G, Giraldi F, Fassini G. Catheter ablation for the treatment of electrical storm in patients with implantable cardioverter-defibrillators: short- and long-term outcomes in a prospective single-center study. Circulation. 2008; 117: 462-469.
- Kozeluhova M, Peichl P, Cihak R, Wichterle D, Vancura V, Bytesnik J. Catheter ablation of electrical storm in patients with structural heart disease. Europace. 2011; 13: 109-113.
- Akihiko Nogami. Trigger elimination of polymorphic ventricular tachycardia and ventricular fibrillation by catheter ablation: trigger and substrate modification. J Biomed Res. 2015; 29: 44-51.
- Soejima K, Suzuki M, Maisel WH, Brunckhorst CB, Delacretaz E, Blier L et al. Catheter ablation in patients with multiple and unstable ventricular tachycardias after myocardial infarction: short ablation lines guided by reentry circuit isthmuses and sinus rhythm mapping. Circulation. 2001; 104: 664-669.
- Patel C, Yan GX, Kocovic D, Kowey PR. Should catheter ablation be the preferred therapy for reducing ICD shocks?: Ventricular tachycardia ablation versus drugs for preventing ICD shocks: role of adjuvant antiarrhythmic drug therapy. CircArrhythmElectrophysiol. 2009; 2: 705-711.
- Arenal A, del Castillo S, Gonzalez-Torrecilla E, Atienza F, Ortiz M, Jimenez J. Tachycardia-related channel in the scar tissue in patients with sustained monomorphic ventricular tachycardias: influence of the voltage scar definition. Circulation. 2004; 110: 2568-2574.