
Case Report
Austin J Clin Cardiolog. 2021; 7(1): 1075.
Myocardial Fibrosis Secondary to Advanced Chagas Disease: A Case Presentation and Review of Literature
Younes A¹, Yalamanchili S¹, Ali H¹, Onyekwelu C² and Movahed A²*
¹Department of Internal Medicine, East Carolina University, USA
²Department of Cardiovascular Sciences, East Carolina University, USA
*Corresponding author: Assad Movahed, East Carolina Heart Institute, 115 Heart Drive, Greenville, NC 27834-4354, USA
Received: March 11, 2021; Accepted: April 01, 2021; Published: April 08, 2021
Abstract
Chagas disease is a systemic infection due to Trypanosoma cruzi, a parasitic protozoan. Trypanosoma cruzi is endemic in Latin America; however, the prevalence has been increasing in the United States. The infection is mostly vector-borne secondary to triatomine or “kissing” bug bites. However, the infection can also spread via organ transplantation, blood transfusion, or transplacentally resulting in congenital manifestations. Chronic Chagas disease can cause cardiac or gastrointestinal complications that may be irreversible if left untreated. Major cardiac complications include dilated cardiomyopathy, arrhythmias, sudden cardiac death, and thromboembolism.
Keywords: Chagas disease; Trypanosoma Cruzi; Heart failure; Myocardial fibrosis; Heart failure with reduced Ejection Fraction (HFrEF); Cardiac MRI (CMRI)
Abbreviations
HFrEF: Heart Failure with Reduced Ejection Fraction; EKG: Electrocardiogram; LVEF: Left Ventricular Ejection Fraction; ARB: Angiotensin Receptor Blocker; CC: Chagas Cardiomyopathy; SCD: Sudden Cardiac Death; ACEIs: Angiotensin Enzyme Inhibitors; VT: Ventricular Tachycardia; ICD: Implantable Cardioverter- Defibrillator
Case Presentation
A 57-year-old Hispanic male with a past medical history of Chagas disease, Heart Failure with reduced Ejection Fraction (HFrEF), and ischemic stroke. The patient presented with dyspnea on exertion for a few days. He described multiple episodes of shortness of breath and chest tightness that last about 30 minutes and improve with rest. He also endorsed bilateral lower extremity nocturnal cramps but denied lower extremity edema, paroxysmal nocturnal dyspnea, cough, nausea, vomiting, abdominal pain, or exertional chest pain.
At the time of presentation, the patient was vitally stable. Cardiac examination revealed the presence of S3 and S4, grade 2-3/6 long systolic murmur best heard over third right and left sternal borders. He had clear breath sounds bilaterally without rhonchi or crackles. Neuro exam was positive for weakness on the left side (which is residual from a previous ischemic stroke).
Pertinent labs: Brain natriuretic peptide was 883pg/ml, D-dimer was 13,185ng/ml. Complete blood count and comprehensive metabolic panel were within normal limits. His lipid panel showed mildly increased LDL and decreased HDL. Troponins were negative.
Five years before this presentation, the patient was admitted due to a right middle cerebral artery ischemic stroke with left-sided hemiparesis. As a part of the work-up, echocardiogram at that time showed dilated cardiomyopathy with an ejection fraction of 40%, and a small apical aneurysm. The patient also gave a history of Chagas disease and reported living in an endemic area. Serology was positive for Trypanosoma cruzi. The patient was thought to be an appropriate candidate for antiparasitic treatment. However, he only showed for one hospital follow-up after this admission and he stopped taking all of his medications.
At the time of the most recent admission, the patient had an x-ray that showed cardiomegaly and bilateral pleural effusions. Electrocardiogram (EKG) showed right bundle branch block and prolonged P-R interval (Figure 1). The patient had a CTA of the chest in the light of a high D-dimer with shortness of breath but it was negative for pulmonary embolism (Figure 2). An echocardiogram on admission showed severely dilated left ventricle, severe global hypokinesis with akinetic to dyskinetic apex, Left Ventricular Ejection Fraction (LVEF) of 15-20 %, and apical linear structure that was suspected to be an LV thrombus (Figure 3).
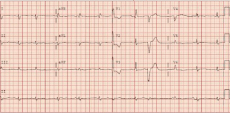
Figure 1: EKG showing right bundle branch block, prolonged PR interval, and
a ventricular premature complex.
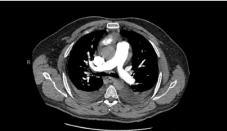
Figure 2: CTA of the chest that is negative for pulmonary emboli.
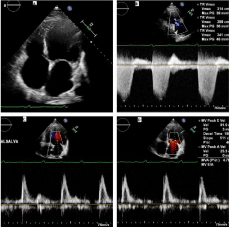
Figure 3A: A: Apical 4-chamber view showing dilated 4 cardiac chambers;
B: Tricuspid velocity indicating a moderate elevation of PASP; C: MV E to
A velocity indicative of restrictive physiology; D: MV E to A with Valsalva
maneuver showing no change in velocity which indicative of irreversible
restrictive physiology.
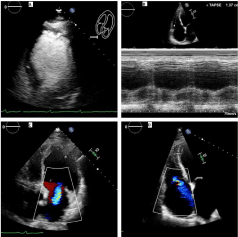
Figure 3B: A: Contrast echo shows severe left ventricular global hypokinesis
and akinetic to dyskinetic apex; B: TAPSE of 1.37 cm indicating moderately
reduced RV function; C: Apical 4-chamber view showing moderate MR; D:
Apical 4-chamber view showing moderate TR.
The patient was admitted with a working diagnosis of acute heart failure exacerbation on top of chronic nonischemic cardiomyopathy due to Chagas disease. He was diuresed with loop diuretics, started on aspirin, high-intensity statin, Angiotensin Receptor Blocker (ARB). The patient experienced an improvement in his shortness of breath with diuresis. Cardiac MRI showed myocardial fibrosis in the form of extensive gadolinium-delayed myocardial contrast enhancement mostly noted in the basal anteroseptal segment. The MRI also confirmed dilated left ventricle, akinesis to dyskinesis of the apex, and the lateral wall with severe hypokinesis in the other regions. There was a small apical aneurysm containing a thrombus. LVEF was estimated to be 19% (Figure 4).
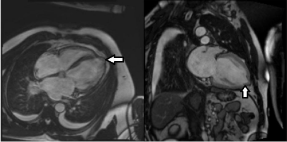
Figure 4A: Cardiac MRI showing dilated LV, apical aneurysm (arrows), and
an LV thrombus.
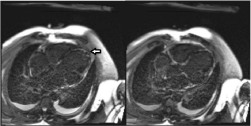
Figure 4B: CMR showing extensive late Gadolinium-contrast myocardial
enhancement (myocardial fibrosis) in the papillary muscle, septal, lateral, and
apical walls of the left ventricle. Apical thrombus is also seen at the LV apex.
Infectious disease service was consulted for further recommendations regarding underlying advanced Chagas disease. Unfortunately, the patient was not a good candidate for antiparasitic treatment (Benzindazole) due to multiple reasons: 1) Antiparasitic treatment is reserved for the early phases of the disease. At the time of presentation, the patient had already developed irreversible myocardial fibrosis. 2) The patient was 57 years old and the use of Benznidazole is associated with an increased risk of toxicity after the age of 50.
After stabilization of the heart failure exacerbation, a beta blocker was initiated. The patient was also started on warfarin. He also received a LifeVest at the time of discharge and was planned to be evaluated for future Implantable Cardioverter-Defibrillator (ICD) placement.
Discussion
Chagas disease was first described in the early 20th Century [1]. It is mainly endemic in Latin America and around 6-8 million people are affected by symptomatic disease [2].
The disease is caused by the protozoan Trypanosoma cruzi and is transmitted through the triatomine Vector (the kissing bug). The amastigotes divide intracellularly, forming trypomastigotes which are released infecting other cells. The protozoan can also be transmitted through organ transplantation, blood transfusion, or transplacental [3]. Acute infection is characterized by nonspecific symptoms such as low-grade fever, generalized malaise, and headaches [4] but it can be rarely complicated with meningitis, encephalitis, or myocarditis.
After the acute phase, most patients become asymptomatic for a long time (up to 30 years). However, asymptomatic patients usually have positive serology and can transmit the disease through blood transfusion or organ donation [5].
Chagas cardiomyopathy
The exact mechanism of Chagas Cardiomyopathy (CC) remains unclear. However, five mechanisms have been proposed [6]:
• Direct invasion of the tissues by the parasite
• Autonomic nervous system dysfunction
• Vasospasm and microvascular disease
• Extracellular matrix remodeling.
• Immune-mediated injury to the myocardium
CC presents mainly with 4 clinical syndromes: Congestive heart failure, Chest pain, Arrhythmias, and Thromboembolism. A correlation between the burden of protozoan infection of cardiomyocytes and the severity of the manifestations has been established [7,8].
Congestive heart failure: Chagas disease causes biventricular failure. However, right-sided heart failure can be more pronounced causing lower extremity edema, hepatomegaly, or ascites. Echocardiographic findings will be explained in a later section [9].
Chest pain: Atypical chest pain is one of the manifestations of CC and it results from myocardial injury and fibrosis or vasospasm due to autonomic instability. It can also be a result of coronary artery disease [10].
Cardiac arrhythmias and sudden cardiac death: CC can present with a wide variety of atrial and ventricular arrhythmias. This can be due to myocardial fibrosis, coronary artery disease, or autonomic instability. Arrhythmias can present with symptoms such as lightheadedness, palpitations, or syncope. Ventricular arrhythmias or complete heart block can result in sudden cardiac death [11]. Sudden Cardiac Death (SCD) secondary to ventricular arrhythmias is common in CC. Some studies have shown the frequency of SCD is up to 60% in untreated CC patients [12].
Thromboembolism: Dilated cardiomyopathy or arrhythmias such as atrial fibrillation can precipitate arterial emboli and stroke in CC patients [13]. In one meta-analysis, Chagas disease patients were found to be more prone to have strokes [14]. Additionally, microvascular disease and atherosclerosis can precipitate arterial thromboembolism [15].
Diagnosis
Patients with confirmed Chagas disease should be assessed for CC. EKG might show signs of cardiac involvement such as bundle branch block, premature atrial or ventricular contractions, or AV block [16].
Chest x-ray might show an enlarged cardiac silhouette due to pericardial effusion or dilated cardiomyopathy [17,18].
Echocardiography might show normal or impaired LVEF, wall motion abnormalities, ventricular aneurysm, or an LV thrombus [19]. Cardiac MRI can also assess the degree of myocardial fibrosis.
Endomyocardial biopsy is reserved to differentiate between transplant rejection (in cardiac transplant patients) and advanced CC. In advanced CC, the biopsy may include myocardial cellular degeneration or fiber hypertrophy and interstitial inflammatory infiltrate associated with edema and fibrosis [20].
Late stages
After acute infection, 70% of the patients go into the indeterminate phase, a latency period characterized by lack of symptoms but with positive serology. This latency period can last between 10-30 years before going into the determinant phase. 30% of the patients go directly into the determinate phase characterized by cardiac and GI symptoms [21].
Chagas disease patients should be assessed regularly with EKGs which can show early signs of cardiac involvement [18]. Patients also should have an echocardiogram every 3-5 years if LVEF is preserved and every 1-2 years if LVEF is reduced [22]. Our patient had worsening of his LVEF from 40% to 20% over the course of five years.
A recent systemic analysis of 5,000 patients showed that the annual rate of cardiomyopathy development was 1.9% in patients with acute Chagas infection [21].
Management of late stages
Antitrypanosomal infection: Nifurtimox and benznidazole have shown efficacy against T. cruzi but benznidazole is considered the first line agent as it is shown to be safer and has more studies to prove efficacy [2]. The benefits of antitrypanosomal therapy in established CC are still controversial [2]. Experts are in agreement to offer benznidazole to individuals who are less than the age of 50 without advanced cardiomyopathy or EKG changes, as prior studies have shown that treatment may decrease the risk of disease progression in indeterminate and chronic CC [23]. However, the recent BENEFIT trial showed that Benznidazole had no significant effect on the progression of CC [24]. It is important to note that those included in this study had an average age of 55 years, and were in the later stages of CC when treatment was initiated.
Congestive heart failure: Heart failure in Chagas disease is treated with the same pharmacological agents used in heart failure of other etiologies and standard therapy includes Angiotensin Enzyme Inhibitors (ACEIs) or ARBs, Beta Blockers, and Diuretics [18]. Aldosterone antagonists and digoxin have also shown some benefit in later stages of HF [25,26]. There are limited randomized controlled trials that have studied the effect of these drugs on cohorts of patients with CC only and evidence is mainly drawn from retrospective analysis of other heart failure studies.
Cardiac transplantation is the last resort in patients with significant functional impairment or inotropic dependence. Interestingly, CC patients had favorable outcomes after cardiac transplantation compared with other etiologies of heart failure despite concerns of reactivation of the disease with immunosuppression [23].
Anticoagulation: Standard anticoagulation therapy should be considered in patients with atrial fibrillation, a history of prior embolism, and intracavitary thrombus [23]. Patients with apical aneurysms with long stalk should also be considered for AC as they are at high risk of forming clots. Agents which have been previously assessed in patients with CC include warfarin and aspirin [18]. Our patient was started on warfarin as he had a left ventricular thrombus, left ventricle aneurysm, and previous stroke.
Cardiac arrhythmias: Pacemaker implantation should be considered in CC patients with Sinus node dysfunction and highgrade AV blocks. Amiodarone is the preferred antiarrhythmic in CC patients with sustained and nonsustained Ventricular Tachycardia (VT) and has shown efficacy in reducing VT episodes, PVCs, and ventricular couplets [27]. ICD is indicated for secondary prevention of life-threatening ventricular arrhythmias and in patients with LVEF <40%. ICDs have shown survival benefit over amiodarone in CC with reduced ejection fraction (LVEF <40%) [27,28]). Our patient didn’t have prior life-threatening arrhythmias but he has significant myocardial fibrosis and severely reduced LVEF so he received a LifeVest at the time of discharge and is being considered for ICD implantation.
Conclusion
CC can have different presentations including arrhythmias, congestive heart failure, and thromboembolic tendency. We present a case of advanced CC with medication nonadherence. Our case is a classic example of almost all the cardiac manifestations of Chagas disease in a relatively young patient.
References
- Chagas disease in Latin America: an epidemiological update based on 2010 estimates. Wkly Epidemiol Rec. 2015; 90: 33-43.
- Rassi A Jr, Rassi A, Marin-Neto JA. Chagas disease. Lancet. 2010; 375: 1388-1402.
- Bern C. Chagas’ Disease. N Engl J Med. 2015; 373: 456-466.
- Castro-Sesquen YE, Gilman RH, Yauri V, Angulo N, Verastegui M, Velásquez DE, et al. Cavia porcellus as a model for experimental infection by Trypanosoma cruzi. Am J Pathol. 2011; 179: 281-288.
- Bern C, Martin DL, Gilman RH. Acute and congenital Chagas disease. Adv Parasitol. 2011; 75: 19-47.
- da Cunha AB. Chagas’ disease and the involvement of the autonomic nervous system. Rev Port Cardiol. 2003; 22: 813-824.
- Bellotti G, Bocchi EA, de Moraes AV, Higuchi M L, Barbero-Marcial M, Sosa E, et al. In vivo detection of Trypanosoma cruzi antigens in hearts of patients with chronic Chagas’ heart disease. Am Heart J. 1996; 131: 301-307.
- Zhang L, Tarleton RL. Parasite persistence correlates with disease severity and localization in chronic Chagas’ disease. J Infect Dis. 1999; 180: 480-486.
- Nascimento CAS, Gomes VAM, Silva SK, Santos CRF, Chambela MC, Madeira FS, et al. Left atrial and left ventricular diastolic function in chronic Chagas disease. J Am Soc Echocardiogr. 2013; 26: 1424-1433.
- Marin-Neto JA, Marzullo P, Marcassa C, Gallo Júnior L, Maciel BC, Bellina CR, et al. Myocardial perfusion abnormalities in chronic Chagas’ disease as detected by thallium-201 scintigraphy. Am J Cardiol. 1992; 69: 780-784.
- Rassi A Jr, Rassi SG, Rassi A. Sudden death in Chagas’ disease. Arq Bras Cardiol. 2001; 76: 75-96.
- de Souza ACJ, Salles G, Hasslocher-Moreno AM, de Sousa AS, Alvarenga Americano do Brasil PE, Saraiva RM, et al. Development of a risk score to predict sudden death in patients with Chaga’s heart disease. Int J Cardiol. 2015; 187: 700-704.
- Carod-Artal FJ, Vargas AP, Falcao T. Stroke in asymptomatic Trypanosoma cruzi infected patients. Cerebrovasc Dis. 2011; 31: 24-28.
- Cardoso RN, Macedo FYB, Garcia MN, Garcia DC, Benjo AM, Aguilar D, et al. Chagas cardiomyopathy is associated with higher incidence of stroke: a meta-analysis of observational studies. J Card Fail. 2014; 20: 931-938.
- Carod-Artal FJ, Gascon J. Chagas disease and stroke. Lancet Neurol. 2010; 9: 533-542.
- Bern C, Montgomery SP, Herwaldt BL, Rassi A, Marin-Neto JA, Dantas RO, et al. Evaluation and treatment of chagas disease in the United States: a systematic review. JAMA. 2007; 298: 2171-2181.
- Parada H, Carrasco HA, Añez N, Fuenmayor C, Inglessis I. Cardiac involvement is a constant finding in acute Chagas’ disease: a clinical, parasitological and histopathological study. Int J Cardiol. 1997; 60: 49-54.
- Andrade JP de, Marin Neto JA, Paola AAV de, Vilas-Boas F, Oliveira GMM, Bacal F, et al. I Latin American Guidelines for the diagnosis and treatment of Chagas’ heart disease: executive summary. Arq Bras Cardiol. 2011; 96: 434-442.
- Pazin-Filho A, Romano MMD, Almeida-Filho OC, Furuta MS, Viviani LF, Schmidt A, et al. Minor segmental wall motion abnormalities detected in patients with Chagas’ disease have adverse prognostic implications. Braz J Med Biol Res. 2006; 39: 483-487.
- Pereira Barretto AC, Mady C, Arteaga-Fernandez E, Stolf N, Lopes EA, Higuchi ML, et al. Right ventricular endomyocardial biopsy in chronic Chagas’ disease. Am Heart J. 1986; 111: 307-312.
- Chadalawada S, Sillau S, Archuleta S, Mundo W, Bandali M, Parra-Henao G, et al. Risk of Chronic Cardiomyopathy Among Patients With the Acute Phase or Indeterminate Form of Chagas Disease: A Systematic Review and Metaanalysis. JAMA Netw Open. 2020; 3: e2015072.
- Nunes MCP, Beaton A, Acquatella H, Bern C, Bolger AF, Echeverría LE, et al. Chagas Cardiomyopathy: An Update of Current Clinical Knowledge and Management: A Scientific Statement from the American Heart Association. Circulation. 2018; 138: e169-209.
- Ribeiro AL, Nunes MP, Teixeira MM, Rocha MOC. Diagnosis and management of Chagas disease and cardiomyopathy. Nat Rev Cardiol. 2012; 9: 576-589.
- Morillo CA, Marin-Neto JA, Avezum A, Sosa-Estani S, Rassi A Jr, Rosas F, et al. Randomized Trial of Benznidazole for Chronic Chagas’ Cardiomyopathy. N Engl J Med. 2015; 373: 1295-1306.
- Khoury AM, Davila DF, Bellabarba G, Donis JH, Torres A, Lemorvan C, et al. Acute effects of digitalis and enalapril on the neurohormonal profile of chagasic patients with severe congestive heart failure. Int J Cardiol. 1996; 57: 21-29.
- Botoni FA, Poole-Wilson PA, Ribeiro ALP, Okonko DO, Oliveira BMR, Pinto AS, et al. A randomized trial of carvedilol after renin-angiotensin system inhibition in chronic Chagas cardiomyopathy. Am Heart J. 2007; 153: 544. e1-e8.
- Stein C, Migliavaca CB, Colpani V, da Rosa PR, Sganzerla D, Giordani NE, et al. Amiodarone for arrhythmia in patients with Chagas disease: A systematic review and individual patient data meta-analysis. PLoS Negl Trop Dis. 2018; 12: e0006742.
- Gali WL, Sarabanda AV, Baggio JM, Ferreira LG, Gomes GG, Marin-Neto JA, et al. Implantable cardioverter-defibrillators for treatment of sustained ventricular arrhythmias in patients with Chagas’ heart disease: comparison with a control group treated with amiodarone alone. Europace. 2014; 16: 674- 680.