
Research Article
Austin J Clin Cardiolog. 2022; 8(1): 1086.
Left Atrial Diameter as a Risk Factor for Atrial Fibrillation Recurrence after Surgical Ablation: A Systematic Review and Meta-analysis
Ye Q¹, Gong Z², Zhao Y¹, Liu K¹, Zhao C¹, Li Y³, Zeng C³ and Wang J¹*
¹Department of Cardiac Surgery, Beijing Anzhen Hospital, Capital Medical University, Beijing, China
²Insitute for Hospital Management of Tsinghua University, Beijing, China
³Center for Cardiac Intensive Care, Beijing Anzhen Hospital, Capital Medical University, Beijing, China
*Corresponding author: Jiangang Wanga, Department of Cardiac Surgery, Beijing Anzhen Hospital, No.2 Anzhen Road, Chaoyang District, Beijing (100029), P.R. China
Received: January 21, 2022; Accepted: February 17, 2022; Published: February 24, 2022
Abstract
Background: Surgical ablation (SA) is widely performed to eliminate atrial fibrillation (AF) and maintain atrial contraction. A larger left atrial diameter (LAD) has long been associated with the late recurrence of AF post-ablation.
Objectives: We conducted a meta-analysis to assess the relationship between LAD and AF recurrence after SA and investigated the effect of LAD cut-off values on the probability of AF recurrence via subgroup analysis.
Methods: The literature search was performed in the MEDLINE and Cochrane Central Register of Controlled Trials databases, from inception to July 2021. A random-effects model was used to estimate the odds ratios (ORs) and 95% confidence intervals (CIs). From 401 initial articles, 16 studies, comprising a total of 4,291 patients, were included in this review.
Results: A meta-analysis of 10 studies (2,599 patients) demonstrated that the predicted probability of AF recurrence was 7% greater with each 1 mm increase in LAD (OR: 1.07; 95% CI: 1.04–1.09; P<0.01). Meanwhile, subgroup analysis revealed that the larger the cut-off value, the higher the risk of AF recurrence. The synthesis effect value (OR: 2.45; 95% CI: 1.77–3.39) was close to the OR when the LAD cut-off value was 55 mm (OR: 2.56; 95% CI: 1.22–5.38).
Conclusions: In conclusion, a larger LAD is a significant risk factor for predicting AF recurrence after SA. More rigorously designed studies with larger sample sizes are needed to identify the best cut-off value of LAD when performing SA.
Keywords: Atrial fibrillation recurrence; Surgical ablation; Left atrial diameter; Meta-analysis
Introduction
Atrial fibrillation (AF) is the most common form of arrhythmia in clinical practice, accounting for approximately one-third of all patients hospitalized due to arrhythmia [1] and is an important contributor to cardiovascular morbidity and mortality [2]. Statistically, AF affects an estimated 2.8% of the general population [3] and 10% of patients undergoing cardiac surgery [4].
Surgical ablation (SA) is performed to eliminate AF and maintain atrial contraction by using surgical lesions to block electrical conduction, which inhibits the generation and propagation of macroreentry circuits in the atria [5,6]. When performed concomitantly with another indicated cardiac surgery, the technique has been shown to reduce the burden of AF on follow-up [7,8]. The lesions created during this procedure are categorized into three groups: pulmonary vein isolation (PVI), left atrial (LA) lesion sets, and biatrial lesion sets [9,10]. Although concomitant ablation of AF during cardiac surgery is beneficial for the maintenance of sinus rhythm (SR), the late recurrence of AF remains a problem [11].
Left atrial diameter (LAD) has long been considered associated with recurrent AF post-ablation [8]. Several studies have confirmed that the larger the LAD, the higher the rate of AF recurrence [12,13]. Specifically, it has been suggested that patients with AF with an LAD >55mm have a significantly increased recurrence rate after catheter ablation conducted according to guidelines and expert consensus [8,14]. Therefore, such patients should be counseled as to the increased risk of operation failure. However, there is inconsistency in the reported threshold LAD value at which AF recurrence after SA occurs [15-18].
Our aim was to conduct a meta-analysis examining the association between LAD and AF recurrence after SA and investigate the effect of LAD cut-off values on the probability of AF recurrence via subgroup analysis.
Methods
This study follows the MOOSE guidelines for meta-analysis reporting [19]. Two investigators searched the MEDLINE and the Cochrane Central Register of Controlled Trials databases, from inception to July 2021. We searched for a combination of English terms and Medical Subject Headings (MeSH) descriptors, consisting of five keywords, as follows: (“surgical ablation” or “maze” or “surgical treatment”) and “atrial fibrillation” and “left atrial.” Each title and abstract was independently analyzed by two investigators who each selected articles relevant to the review. Subsequently, the full texts of the remaining articles were reviewed to select which would be included in the qualitative and quantitative analyses. In case of disagreement, a third investigator joined the discussion and made the decision.
Studies were included if they met the following criteria: (1) evaluated AF recurrence after SA in human participants; (2) measured the association between LAD and AF recurrence; (3) included no less than 50 participants; and (4) had a mean/median follow-up duration of more than 6 months.
Studies were ineligible if they did not report the odds ratio (OR)/ hazard ratio (HR) and the 95% confidence interval (CI) of LAD as a risk factor for AF recurrence. Furthermore, studies were excluded if LAD was reported in centimeters. When institutions published duplicate reports of a study, with accumulating number of patients or increased follow-up durations, only the most complete reports were included for quantitative assessment. For the subgroup analysis, only articles that fulfilled all the previous criteria and reported OR/HR and 95% CI of LAD at each threshold were included.
Data extraction was performed using a standard form by two investigators and cross-verified by a third. Extracted data included (1) first author’s last name, publication year, and country; (2) study characteristics, specifically number of patients, study design, lesion set, energy, definition of AF recurrence, and method of AF detection; and (3) outcome results, specifically OR/HR and 95% CI of LAD in multivariate analysis, and endpoint rates (including overall death, SR, stroke, and pacemaker insertion) at the final follow-up.
The risk of bias in the studies was evaluated using the National Heart, Lung and Blood Institute Quality Assessment Tool for Case Series Studies [20], which rate studies as “good,” “fair,” or “poor.” The evaluation was done independently by two raters, and in case of disagreement, a third rater joined the discussion and made the decision. The quality assessment of the included studies is reported in Table 1.
First Author
Year
Sample, n
Design
Lesion set
Energy
Surgery type
Definition of AF recurrence
Monitoring
Follow-up, months
Primary outcome of the last follow-up
Cut-off value of LAD, mm
Quality
Kamata et al. [1]
1997
96
Retrospective, single-center
BA
CS+CY
MV, AV, CABG, CHD, and COMB-
Persistent AF and paroxysmal AF
Ambulatory electrocardiographic monitoring and ECG
Not less than 6
Death:4.2% SR:79.1% Stroke:NR PM:6.5%
65
Good
Baek et al. [2]
2006
170
Retrospective, single center
BA
CS+CY
MV +/- ( AV or TV or CABG)
Documented episodes of AF or atrial flutter
ECG and 24h-Holter monitoring
26.6 (mean)
Death:2.4% SR:82.9% Stroke:2.4% PM:1.8%
65
Good
Grubitzsch et al. [3]
2007
212
Retrospective, single center
LA
MW, RF
MV, AV, CABG, and COMB-
Documented episodes of AF or atrial flutter
ECG and 24h-Holter monitoring
13 (mean)
Death:7.1% SR:70.7% Stroke:2.4% PM:3.3%
NR
Good
Melo et al. [4]
2008
972
Retrospective, multicenter
BA, LA, PVI
RF, MW, CY
MV +/- ( AV or TV or CABG or other)
Documented episodes of AF or atrial flutter
ECG or 24h-Holter monitoring
29 (mean)
Death:6.6% SR:66% Stroke:3% PM:3%
55
Good
Beukema et al. [5]
2008
285
Retrospective, single center
BA
RF
MV, AV, CABG, and COMB-
Atrial flutter/atrial tachycardia or AF
ECG or 24h-Holter monitoring
43.6 (mean)
Death:27.4% SR:56% Stroke:2.1% PM:NR
60
Good
Je et al. [6]
2009
560
Retrospective, single-center
BA
CY, MW
MV, AV, TV, CABG, and COMB-
Documented episodes of AF or atrial flutter
ECG or 24h-Holter monitoring
29.7 (median)
Death:3.8% SR:84.1% Stroke:1.3% PM:2.3%
60
Good
Funatsu et al. [7]
2009
268
Retrospective, single center
BA
CY
MV +/- ( AV or TV or CABG)
Documented episodes of AF or atrial flutter
ECG or Holter ECG
45.6 (mean)
Dearth:1.9% SR:80.2% Stroke:NR PM:8.3%
70
Good
Kim et al. [8]
2010
435
Retrospective, single center
BA
CY, MW
MV +/- ( AV or TV or CABG or other)
Atrial flutter/atrial tachycardia or AF
ECG or 24h-Holter monitoring
40.6(median)
Death:4.0% SR:82.7% Stroke:1.4% PM:2.3%
60
Good
Kainuma et al. [9]
2013
50
Retrospective, single center
BA
CY
MV +/- ( AV or TV or CABG)
Rapid irregular rhythm with disorganized atrial activity
ECG or 24h-Holter monitoring, echo
59 (mean)
Death:6% SR:78% Stroke:2% PM:16%
60
Good
Dong et al. [10]
2013
191
Retrospective, single center
BA
RF
MV +/- ( AV or TV )
Episode of AF, atrial flutter, or atrial tachycardia that lasted more than 30 seconds
ECG or 24h-Holter monitoring
43.7 (mean)
Death:1.6% SR:79.11% Stroke:0% PM:0%
60
Good
Choi et al. [11]
2013
89
Retrospective, single center
BA
CS+CY, RF+CY
MV, AV, CABG, CHD, and COMB-
NR
ECG or 24h-Holter monitoring
51.0 (mean)
Death:NR SR:88.8% Stroke:NR PM:2.2%
NR
Good
Tsai et al. [12]
2015
287
Retrospective, single-center
BA
RF+CY
MV, AV, CABG, TV, and COMB-
Episode of AF, atrial flutter, or atrial tachycardia that lasted more than 30 seconds
ECG and 24h-Holter monitoring
38.0 (mean)
Death:NR SR:75.8% Stroke:NR PM:NR
NR
Good
Kainuma et al. [13]
2015
160
Retrospective, single center
PVI
RF
AV, CABG, and COMB-
Atrial flutter/atrial tachycardia or AF
ECG and 24h-Holter monitoring
47 (mean)
Death:5.3% SR:85% Stroke:2.4% PM:1.4%
45
Good
Wu et al. [14]
2017
207
Retrospective, single center
BA, LA
RF
MV +/- ( AV or TV or CHD)
NR
ECG or 24h-Holter monitoring
101 (mean)
Death:8.2% SR:74.4% Stroke:1.4% PM:3.9%
59.85
Good
Pyo et al. [15]
2019
146
Retrospective, single center
BA, LA
CY, MW
MV and/or AV, +/- ( TV or CABG)
Episode of AF, atrial flutter, or atrial tachycardia that lasted more than 30 seconds
ECG and 24h-Holter monitoring
22.5 (mean)
Death:19.4% SR:59.8% Stroke:2.2% PM:6.5%
57.5
Good
Raissouni et al. [16]
2019
163
Retrospective, single center
LA, PVI
RF
MV, AV, CABG, and COMB-
NR
ECG or 24h-Holter monitoring
Not less than 6
Death:5.3% SR:61% Stroke:2.1% PM:2.7%
40
Good
AF: Atrial Fibrillation; AV: Aortic Valve Surgery; BA: Biatrial; CABG: Coronary Artery Bypass Grafting; COMB: Combinations; CS: Cut and Sew; CY: Cryoablation; Echo, Echocardiography; ECG: Electrocardiograph; LA: Left Atrial; MV: Mitral Valve Surgery; MW: Microwave; NR: Not Reported; PM: Pacemaker; PVI: Pulmonary Vein Isolation; RF: Radiofrequency; SR: Sinus Rhythm; TV: Tricuspid Valve Surgery.
Table 1: Characteristics of the included studies.
The association between AF recurrence after SA and LAD was measured using OR/HR and 95% CIs. The log of each OR/HR was obtained by calculating the natural logarithm. Standard errors were determined from the logarithmic scale and corresponding 95% CIs. The inverse variance method was used to weigh studies according to the combined overall statistics. Statistical significance was defined as p < 0.05. Heterogeneity between studies was assessed using the Cochran’s Q test and I² statistic and then evaluated using I2 values. The random-effects model was chosen because of the different lesion sets, which could lead to heterogeneity. Sensitivity analysis was performed by excluding studies and checking the consistency of the overall effect estimate. The results are presented in a forest plot with 95% CIs. Publication bias was verified using a funnel plot. Possible asymmetry was investigated using trim-and-fill analysis [21]. All analyses were performed using Review Manager (version 5.3) and R statistical package (version 3.6.1).
Results
Initially, a total of 401 articles were identified across the two databases: 331 in MEDLINE and 70 in the Cochrane Central Register of Controlled Trials. We identified 48 duplicate articles, which were subsequently excluded. We screened the resulting 353 studies and excluded 311 that did not fulfill the eligibility criteria based on the review of the title and abstract and reviewed the full texts of the remaining 42 studies and identified 26 that were not eligible for inclusion. Thus, 16 studies were included in the qualitative analysis and 10 in the meta-analysis. The study selection process is illustrated in Figure 1.
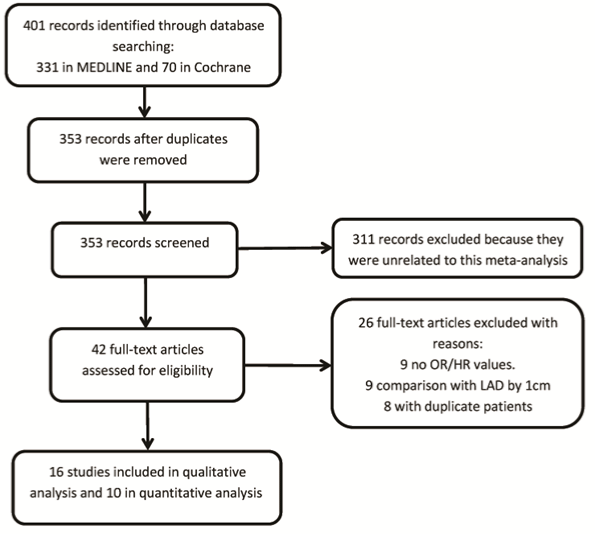
Figure 1: Flow diagram of the study selection. HR: Hazard Ratio; LAD: Left Atrial Diameter; OR: Odds Ratio.
Table 1 summarizes the general characteristics of the 16 studies. Data on 4,291 patients were reported across the 16 studies [15,16,18,22-34], which were all retrospective single-center cohort studies, except that by Melo et al. [26], which was a multicenter study. The mean follow-up duration ranged from 1 to 10 years. Holter monitoring was performed in all studies to diagnose AF. Fifteen studies included biatrial lesions or LA lesions in their protocol, while one study by Kainuma et al. [31], performed only the PVI procedure because the included patients had undergone concomitant aortic valve replacement and/or coronary artery bypass grafting. The results of these studies were satisfactory, with SR rates ranging from 56% to 88.8% and stroke rates ranging from 0% to 3% at the final follow-up.
Our meta-analysis of 10 studies [24-26,28-34] (2,599 patients) identified larger LAD to be associated with a higher AF recurrence after (OR: 1.07; 95% CI: 1.04-1.09; P<0.01, Figure 2), meaning that the predicted probability of AF recurrence increased by 7% with each 1 mm increase in LAD. The heterogeneity test indicated significant differences between studies (P<0.01, I² = 67%). The sensitivity analysis, performed to determine the origin of the heterogeneity, revealed that after removing the Kainuma et al. study [31], which only used the PVI protocol, and the Pyo et al. study [34], which only included patients aged over 60 years who had undergone bioprosthetic valve replacement, no significant heterogeneity across the studies remained (P>0.05, I² = 48%). Nevertheless, the overall outcome remained the same (OR: 1.07; 95% CI: 1.05-1.10; P<0.01). Visual inspection of a funnel plot confirmed the presence of publication bias (Figure 3). Using the imputed trim-and-fill method, we found that three studies were estimated to be “missing,” with the point estimate adjusted slightly from 1.07 (95% CI: 1.04-1.09) to 1.06 (95% CI: 1.04-1.08).
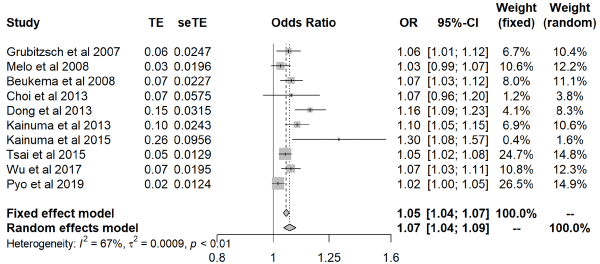
Figure 2: Forest plot showing left atrial diameter as a predictor of atrial fibrillation recurrence after surgical ablation.
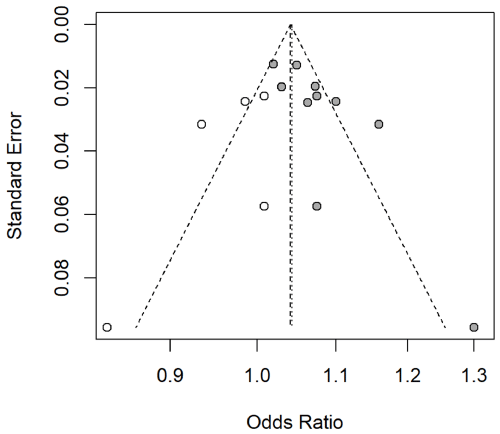
Figure 3: Risk of bias funnel plot.
Across the studies, there was disagreement over the cut-off value of LAD associated with AF recurrence after SA. Because different patients, lesion sets, ablation energies, and especially statistical methods were used, we could not identify the best cut- off value of LAD beyond which the risk of AF recurrence increased significantly. Hence, we conducted a subgroup analysis to assess the association between LAD, measured as a dichotomous variable, and AF recurrence after SA. Eight studies [15,16,18,22,23,26,27,30] were included in the subgroup analysis, which found that the larger the cut-off value, the higher the risk of AF recurrence after SA. In the random-effects model (Figure 4), the synthesis effect value (OR: 2.45; 95% CI: 1.77-3.39) was close to the OR value when the LAD cut-off value was 55mm (OR: 2.56; 95% CI: 1.22-5.38). The heterogeneity test did not identify any significant difference between the studies (P=0.22, I2= 26%).
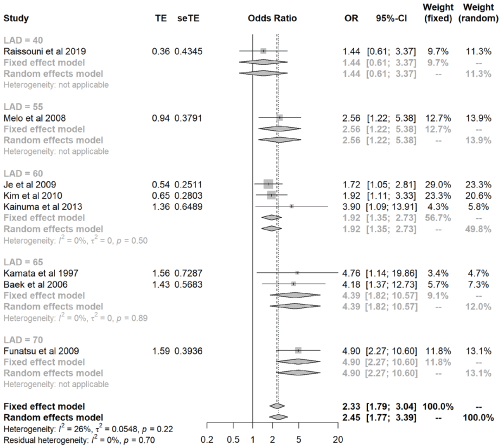
Figure 4: Subgroup analysis shows that the larger the cut-off value, the higher the risk of AF recurrence after SA, and the synthesis effect value is close to the
OR value when the LAD cut-off value is 55mm.
AF: Atrial Fibrillation; LAD: Left Atrial Diameter; OR: Odds Ratio; SA: Surgical Ablation.
Discussion
Surgical AF ablation is widely performed worldwide to concomitantly treat AF in cardiac surgery, although it does not resolve AF in all patients, some of whom experience late recurrence of AF [35-37]. This meta-analysis first quantitatively demonstrated that, despite some limitations, a larger LAD was a strong risk factor for AF recurrence after SA, with a 7% greater probability of recurrence with each 1mm increase in LAD. A previous review found that the mean preoperative LAD among patients with AF was consistently over 60 mm and that the relationship between preoperative LAD and failure of the maze procedure appeared continuous; the authors thus concluded that patients should be counseled as to the increased risk of failure as they increase above an LAD of 60mm [38]. This was similar to the result of our subgroup analysis showing that the risk of AF recurrence increased over a cut-off value greater than 60mm.
LA enlargement is usually caused by excessive atrial load, which leads to stretching of the atrial wall. Atrial stretch activates the renin-angiotensin-aldosterone system, which generates multiple downstream profibrotic factors, including transforming growth factor-beta 1, and then promotes atrial fibrosis. Extensive cardiomyocyte-fibroblast electric interaction, with the induction of reentry and spontaneous ectopic activity, is an important contributor to the AF substrate [39-41]. Thus, it can be inferred that an enlarged left atrium is closely associated with AF recurrence post-ablation.
Patients with AF with an LAD >55mm have significantly higher recurrence rates after catheter ablation [14,42]. However, there is still disagreement over the most appropriate cut-off value of LAD associated with AF recurrence after SA. For example, Feyrer et al. [43] analyzed 103 patients (78 with LAD <50mm and 25 with LAD >50mm) undergoing SA and found that 67% of those with LAD <50mm were successfully converted to SR by 3 months post-ablation, while only 48% of patients with LAD >50mm were successfully converted. Meanwhile, Vural et al. [44] found that LAD was associated with AF recurrence and that the sensitivity and specificity associated with an LAD cut-off value of 50.5mm for the maintenance of SR were 85.7% and 70.7%, respectively. In addition, some studies have described the association between an LAD cut-off value of 60mm and AF recurrence after SA, and all confirmed that LAD >60mm was a reasonable predictor of AF recurrence after SA [25,45-47]. Otherwise, whether LA size reduction improves SA success is controversial. Yalcinkaya et al. [48] used the posterior LA wall plication technique for LA size reduction in patients undergoing mitral valve surgery and achieved satisfactory results in terms of mid-term restoration and preservation of normal SR, while Damiano et al. [49] reported no benefit of atrial reduction plasty in patients with an LAD >70mm.
Additionally, other factors such as age, longer preoperative AF duration, and persistent AF are associated with the late recurrence of AF, and the selection of different patients and lesion sets may affect the long-term efficacy of SA [8,50]. Nevertheless, considering that LAD can be measured more accurately and easily, we believe that a more reliable threshold value of LAD is still needed for the evaluation of patients undergoing SA in clinical practice. The results of our subgroup analysis indicated that an LAD cut-off of 55mm might also be applied for surgical ablation, considering that the risk of SA failure significantly increased at that threshold. Further well-designed cohort studies should be conducted to verify this conclusion. Furthermore, Kim et al. [51] suggested that the addition of the maze procedure in patients with AF undergoing mechanical mitral valve replacement was associated with reduced thromboembolic complications and improved long-term event-free survival. However, whether these patients could benefit from SA when presenting an LAD >55mm remains unclear. Especially, two-thirds of the world’s population live in developing countries with a high prevalence of rheumatic fever or rheumatic heart disease [52], resulting in a large population with mitral stenosis combined with a larger LAD above 55mm as a result of poverty and late medical treatment. Since these patients undergoing mechanical valve replacement have significant recurrence after ablation, whether SA is obligatory given the surgical cost remains controversial. Further studies are warranted to identify an effective surgical strategy for patients with AF undergoing mechanical valve replacement with an LAD > 55mm.
Studies included in this meta-analysis used various definitions of AF recurrence, ranging from paroxysmal or persistent AF to any episode of AF, atrial flutter, or atrial tachycardia lasting more than 30sec. Ablation strategy and patient selection across the studies also varied. Combined, this caused heterogeneity. In addition, although adjusted OR/HR values from multivariate analyses were used to reduce the effects of confounding variables, their influence could not be excluded completely. Therefore, despite considerable evidence for an increased risk of AF recurrence with LA enlargement, further studies are needed to better understand the relationship between LAD and AF and identify a reliable cut-off value of LAD when performing SA.
Conclusions
In conclusion, LAD is a significant risk factor for AF recurrence after SA. The larger the preoperative LAD, the higher the probability of AF recurrence. More rigorously designed studies with larger sample sizes are needed to identify the most reliable cut-off value of LAD when performing SA.
Acknowledgments
This work was supported in part by grants from the National Natural Science Foundation of China (no. 81770320).
References
- Chugh SS, Havmoeller R, Narayanan K, et al. Worldwide epidemiology of atrial fibrillation: a Global Burden of Disease 2010 Study. Circulation. 2014; 129: 837-847.
- Benjamin EJ, Wolf PA, D’Agostino RB, et al. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation. 1998; 98: 946-952.
- Virani SS, Alonso A, Benjamin EJ, et al. Heart Disease and Stroke Statistics-2020 Update: A Report From the American Heart Association. Circulation. 2020; 141: e139-139e596.
- Ball J, Carrington MJ, McMurray JJ, et al. Atrial fibrillation: profile and burden of an evolving epidemic in the 21st century. Int J Cardiol. 2013; 167: 1807- 1824.
- Cox JL, Schuessler RB, D’Agostino HJ Jr., et al. The surgical treatment of atrial fibrillation. III. Development of a definitive surgical procedure. J Thorac Cardiovasc Surg. 1991; 101: 569-583.
- Toeg HD, Al-Atassi T, Lam BK. Atrial fibrillation therapies: lest we forget surgery. Can J Cardiol. 2014; 30: 590-597.
- Gillinov AM, Gelijns AC, Parides MK, et al. Surgical ablation of atrial fibrillation during mitral-valve surgery. N Engl J Med. 2015; 372: 1399-1409.
- Calkins H, Hindricks G, Cappato R, et al. 2017 HRS/EHRA/ECAS/APHRS/ SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Europace. 2018; 20: e1-e160.
- Ad N, Damiano RJ Jr., Badhwar V, et al. Expert consensus guidelines: Examining surgical ablation for atrial fibrillation. J Thorac Cardiovasc Surg. 2017; 153: 1330-1354.e1.
- McClure GR, Belley-Cote EP, Jaffer IH, et al. Surgical ablation of atrial fibrillation: a systematic review and meta-analysis of randomized controlled trials. Europace. 2018; 20: 1442-1450.
- Prystowsky EN, Padanilam BJ. Treatment of atrial fibrillation: a weighty problem. J Am Coll Cardiol. 2015; 65: 2170-2172.
- Ad N, Holmes SD, Massimiano PS, et al. Long-term outcome following concomitant mitral valve surgery and Cox maze procedure for atrial fibrillation. J Thorac Cardiovasc Surg. 2018; 155: 983-994.
- Yang S, Mei B, Feng K, et al. Long-Term Results of Surgical Atrial Fibrillation Radiofrequency Ablation: Comparison of Two Methods. Heart Lung Circ. 2018; 27: 621-628.
- January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation. 2014; 130: 2071-2104.
- Funatsu T, Kobayashi J, Nakajima H, et al. Long-term results and reliability of cryothermic ablation based maze procedure for atrial fibrillation concomitant with mitral valve surgery. Eur J Cardiothorac Surg. 2009; 36: 267-271; discussion 271.
- Kim JB, Yun TJ, Chung CH, et al. Long-term outcome of modified maze procedure combined with mitral valve surgery: analysis of outcomes according to type of mitral valve surgery. J Thorac Cardiovasc Surg. 2010; 139: 111-117.
- Ad N, Henry L, Hunt S, et al. Should surgical ablation for atrial fibrillation be performed in patients with a significantly enlarged left atrium? J Thorac Cardiovasc Surg. 2014; 147: 236-241.
- Raissouni K, Petrosyan A, Malapert G, et al. Concomitant Cardiac Surgery and Radiofrequency Ablation of Atrial Fibrillation: A Retrospective Single Center Study. J Cardiothorac Vasc Anesth. 2020; 34: 401-408.
- Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000; 283: 2008-2012.
- National Heart, Lung, and Blood Institute. NIH. Study Quality Assessment Tools [Internet]. Bethesda: NIH. 2018.
- Duval S, Tweedie R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000; 56: 455-463.
- Kamata J, Kawazoe K, Izumoto H, et al. Predictors of sinus rhythm restoration after Cox maze procedure concomitant with other cardiac operations. Ann Thorac Surg. 1997; 64: 394-398.
- Baek MJ, Na CY, Oh SS, et al. Surgical treatment of chronic atrial fibrillation combined with rheumatic mitral valve disease: Effects of the cryo-maze procedure and predictors for late recurrence. Eur J Cardiothorac Surg. 2006; 30: 728-736.
- Grubitzsch H, Dushe S, Beholz S, et al. Surgical ablation of atrial fibrillation in patients with congestive heart failure. J Card Fail. 2007; 13: 509-516.
- Beukema WP, Sie HT, Misier AR, et al. Predictive factors of sustained sinus rhythm and recurrent atrial fibrillation after a radiofrequency modified Maze procedure. Eur J Cardiothorac Surg. 2008; 34: 771-775.
- Melo J, Santiago T, Aguiar C, et al. Surgery for atrial fibrillation in patients with mitral valve disease: results at five years from the International Registry of Atrial Fibrillation Surgery. J Thorac Cardiovasc Surg. 2008; 135: 863-869.
- Je HG, Lee JW, Jung SH, et al. Risk factors analysis on failure of maze procedure: mid-term results. Eur J Cardiothorac Surg. 2009; 36: 272-278; discussion 278-279.
- Choi JB, Park HK, Kim KH, et al. Predictive factors of sustained sinus rhythm and recurrent atrial fibrillation after the maze procedure. Korean J Thorac Cardiovasc Surg. 2013; 46: 117-123.
- Dong L, Fu B, Teng X, et al. Clinical analysis of concomitant valve replacement and bipolar radiofrequency ablation in 191 patients. J Thorac Cardiovasc Surg. 2013; 145: 1013-1017.
- Kainuma S, Funatsu T, Kondoh H, et al. Novel surgical ablation through a septal-superior approach for valvular atrial fibrillation: 7-year single-centre experience. Eur J Cardiothorac Surg. 2013; 44: 1013-1022; discussion 1022.
- Kainuma S, Mitsuno M, Toda K, et al. Dilated left atrium as a predictor of late outcome after pulmonary vein isolation concomitant with aortic valve replacement and/or coronary artery bypass grafting†. Eur J Cardiothorac Surg. 2015; 48: 765-777; discussion 777.
- Tsai FC, Ho HT, Chang JP, et al. The Prognostic Scoring System Establishment and Validation for Chronic Atrial Fibrillation Patients Receiving Modified Cox-Maze IV and Concomitant Cardiac Surgery. PLoS One. 2015; 10: e0126300.
- Wu CC, Chang JP, Chen MC, et al. Long-term results of radiofrequency maze procedure for persistent atrial fibrillation with concomitant mitral surgery. J Thorac Dis. 2017; 9: 5176-5183.
- Pyo W, Park SJ, Kim WK, et al. Surgical Ablation of Atrial Fibrillation in Patients Undergoing Bioprosthetic Valve Replacement. Korean J Thorac Cardiovasc Surg. 2019; 52: 61-69.
- Wokhlu A, Monahan KH, Hodge DO, et al. Long-term quality of life after ablation of atrial fibrillation the impact of recurrence, symptom relief, and placebo effect. J Am Coll Cardiol. 2010; 55: 2308-2316.
- Phan K, Xie A, La Meir M, et al. Surgical ablation for treatment of atrial fibrillation in cardiac surgery: a cumulative meta-analysis of randomized controlled trials. Heart. 2014; 100: 722-730.
- Badhwar V, Rankin JS, Damiano RJ Jr., et al. The Society of Thoracic Surgeons 2017 Clinical Practice Guidelines for the Surgical Treatment of Atrial Fibrillation. Ann Thorac Surg. 2017; 103: 329-341.
- Sunderland N, Maruthappu M, Nagendran M. What size of left atrium significantly impairs the success of maze surgery for atrial fibrillation? Interact Cardiovasc Thorac Surg. 2011; 13: 332-338.
- Burstein B, Nattel S. Atrial fibrosis: mechanisms and clinical relevance in atrial fibrillation. J Am Coll Cardiol. 2008; 51: 802-809.
- Nattel S, Burstein B, Dobrev D. Atrial remodeling and atrial fibrillation: mechanisms and implications. Circ Arrhythm Electrophysiol. 2008; 1: 62-73.
- Andrade J, Khairy P, Dobrev D, et al. The clinical profile and pathophysiology of atrial fibrillation: relationships among clinical features, epidemiology, and mechanisms. Circ Res. 2014; 114: 1453-1468.
- Prystowsky EN, Padanilam BJ, Fogel RI. Treatment of Atrial Fibrillation. JAMA. 2015; 314: 278-288.
- Feyrer R, Ballazhi F, Seitz T, et al. Impact of medical treatment on long-term results after surgical ablation of atrial fibrillation in cardiac surgical patients. Ann Thorac Cardiovasc Surg. 2014; 20: 207-212.
- Vural Ü, Balci AY, Aglar AA, et al. Which Method to Use for Surgical Ablation of Atrial Fibrillation Performed Concomitantly with Mitral Valve Surgery: Radiofrequency Ablation versus Cryoablation. Braz J Cardiovasc Surg. 2018; 33: 542-552.
- Chaiyaroj S, Ngarmukos T, Lertsithichai P. Predictors of sinus rhythm after radiofrequency maze and mitral valve surgery. Asian Cardiovasc Thorac Ann. 2008; 16: 292-297.
- Ghavidel AA, Javadpour H, Shafiee M, et al. Cryoablation for surgical treatment of chronic atrial fibrillation combined with mitral valve surgery: a clinical observation. Eur J Cardiothorac Surg. 2008; 33: 1043-1048.
- Pecha S, Schäfer T, Subbotina I, et al. Rhythm outcome predictors after concomitant surgical ablation for atrial fibrillation: a 9-year, single-center experience. J Thorac Cardiovasc Surg. 2014; 148: 428-433.
- Yalcinkaya A, Diken AI, Aksoy E, et al. Effect of Left Atrial Reduction on Restoration and Maintenance of Sinus Rhythm in Patients Undergoing Mitral Valve Replacement: A Pilot Study. Thorac Cardiovasc Surg. 2016; 64: 441- 446.
- Damiano RJ Jr., Schwartz FH, Bailey MS, et al. The Cox maze IV procedure: predictors of late recurrence. J Thorac Cardiovasc Surg. 2011; 141: 113-121.
- Khiabani AJ, MacGregor RM, Bakir NH, et al. The long-term outcomes and durability of the Cox-Maze IV procedure for atrial fibrillation. J Thorac Cardiovasc Surg. 2020.
- Kim JB, Ju MH, Yun SC, et al. Mitral valve replacement with or without a concomitant Maze procedure in patients with atrial fibrillation. Heart. 2010; 96: 1126-1131.
- Chandrashekhar Y, Westaby S, Narula J. Mitral stenosis. Lancet. 2009; 374: 1271-1283.