
Research Article
Austin J Clin Cardiolog. 2022; 8(1): 1088.
Allograft Vasculopathy in Transplanted Hearts and the Role of FGF 23-Klotho Axis
Evlice M*
Department of Cardiology, Bingol State Hospital, Bingol, Turkey
*Corresponding author: Mert Evlice, Department of Cardiology, Bingol State Hospital, 12000 Bingol, Turkey
Received: February 18, 2022; Accepted: March 12, 2022; Published: March 19, 2022
Abstract
Background: There are increasing evidences in the role of the involvement of the fibroblast growth factor 23 (FGF 23) - clotho axis in the pathogenesis of endothelial disfunction and cardiovascular disease. This study intended to explore the role of FGF 23 - clotho axis in the development of allograft vasculopathy.
Methods: A total of 38 biatrial heart transplant patients who were operated were included in the study (20 males, 11 females; mean age: 44 ± 7 years). CFR was measured in all patients and the patients were divided into two groups according to respective CFR values. CFR > 2 patients constituted CAV (-) group, CFR < 2 patients were enrolled into CAV (+) group. FGF 23 and clotho levels were analyzed and compared in both groups.
Results: FGF 23 levels were significantly higher in CAV (+) group (264.0±114.4 vs. 183.5±56.0 p=0.04). There was a good but inverse correlation between CFR and FGF 23 levels in CAV (+) group (r= - 0.71 p=0.03). Clotho levels were significantly lower in patients who have CAV (2.76±1.6 vs. 4.77±0.87 p=0.01). There was a moderate correlation between CFR and clotho levels in CAV (+) group (r=0.62 p=0.04). There was an inverse correlation between clotho and FGF 23 levels in both CAV (+) and CAV (-) groups.
Conclusion: In transplanted patients, there was a good but negative correlation between CFR and FGF 23 levels. Conversely, there was a good correlation between CFR and clotho levels. These results gave rise to the thought clotho-FGF 23 axis has a role in the development of CAV.
Keywords: Fibroblast growth factor 23; Cardiac allograft vasculopathy; Nitric oxide
Introduction
Accelerated arteriosclerosis has emerged as a major lifethreatening complication in long-term survivors of cardiac transplantation. Both cardiac allograft vasculopathy (CAV) and atherosclerosis are atheromatous diseases with some common features, but there are also many distinctive characteristics of both diseases such as sites and degree of involvement, progression rate and more prominent autoimmune involvement in transplanted hearts. Conventional risk factors such as diabetes, hypertension, age, smoking and chronic kidney disease partly, but not entirely explain the increase in morbidity and mortality. Despite successful interventions on traditional risk factors, risk remains high in cardiovascular disease. Endothelial dysfunction is regarded as an important contributor to increased cardiovascular risk [1].
Endothelial dysfunction is a systemic pathological condition which can be defined as a condition resulting from an imbalance between the actions of vasorelaxing and vasoconstrictor factors. The imbalance is mainly caused by reduced nitric oxide (NO) bioavailability and/or increased generation of reactive oxygen species (ROS) [2].
Fibroblast Growth Factor 23 (FGF 23) is a mutated gene identified in autosomal dominant hypophosphatemic rickets with 251-amino acid residuals in the protein [3]. The FGF family has 23 proteins that regulate cell proliferation, migration, differentiation and survival. Several known subgroups of human FGFs have been defined. The FGF19 subfamily comprises FGF19, FGF21 and FGF 23. FGF 23 is produced by osteocytes, and regulates phosphate homeostasis via FGFR1 receptor signaling in the presence of klotho. Klotho gene represents a type I single-pass transmembrane protein that is associated with Β-glucuronidases. Membrane klotho interacts with FGF receptors (especially FGFR1) to form a high-affinity for FGF 23, stimulates phosphate excretion into the urine and decreases the level of serum 1,25(OH)2D3, and inhibits secretion of parathyroid hormone. Secreted klotho protein functions as a humoral factor that modifies several ion channels and transporters, and other processes, including insulin and insulin-like growth factor-1 signaling. Soluble klotho also plays an important role in the regulation of NO production and the integrity and permeability of endothelium [4]. In cooperation with klotho, FGF 23 regulates blood calcium level by suppressing the synthesis of 1,25(OH)D3 and reabsorption of phosphate in the proximal convoluted part of the nephron. FGF 23 also can negatively regulate the secretion of parathyroid hormone [5].
There are also increasing evidences in the role of the involvement of the FGF 23-klotho-vitamin D axis in the pathogenesis of endothelial disfunction and cardiovascular disease. Klotho and FGF 23 may function in a common single transduction pathway to accelerate vascular calcification and cardiac hypertrophy. Klotho-null mice or FGF 23-deficient mice shows early atherosclerosis, vascular calcifications, impaired angiogenesis, and vasculogenesis, suggesting the impact of this pairing on the pathophysiology of cardiovascular disorders [6].
In this study, we aimed to explore the role of FGF 23-klotho axis in allograft vasculopathty in patients with heart transplantation.
Methods
Study population
A total of 52 heart transplant patients were included in the study (36 males, 20 females; mean age: 46.5 ± 9.7 years). 4 patients were excluded due to poor echo imaging, unsatisfactory coronary flow reserve (CFR) measurements or meeting other exclusion criteria. 50 eligible patients continued to the study. Study protocol was designed as prospective clinical cohort study. Baseline CFR measurement were done in the first or second month postoperatively. 2 patients with abnormal baseline CFR results (CFR ≤ 2) were excluded. 46 patients with normal baseline CFR results (CFR > 2) continued study. 8 patients developed antibody mediated rejection (AMR) or cell mediated rejection (CMR) (5 AMR and 3 CMR) during follow up. Initially, those patients excluded from main cohort to prevent overlapping results. 6 of those were evaluated (4 AMR and 2 CMR) after completion of study. After median 4 years follow up, CFR and FGF 23-Klotho analyses were done. CAV (+) group consisted of patients with CFR ≤ 2. CAV (-) group consisted of patients with CFR ≤ 2. During follow up, Coronary angiography was performed in 12 individuals out of 38 patients. Patients were followed median four years. At the end of at least 1 year follow up period, CFR and FGF 23-klotho values were measured.
Patients with moderate to severe left ventricular (LV) wall motion abnormality, rejection, LV ejection fraction (EF) less than 50%, atrioventricular conduction abnormalities on ECG, pericarditis, thyroid dysfunction (TSH >4.0mIU/l or < 0.4mIU/l) , anemia (Hb < 13gr/dl) hypercholesterolemia (LDL > 190mg/dl) , electrolyte imbalance (Na levels greater than 145mEq/l or lower than 135mEq/l, K levels greater than 3.5mEq/l or lower than 5mEq/l, calcium levels greater than 8.5mg/dl or lower than 10.5mg/dl ), renal dysfunction (glomerular filtration rate lower than 90ml/min), pulmonary disease (obstructive physiology defined as FEV1/FVC < 70% or reduced vital capacity and/or reduced total lung volume), moderate to severe valvular dysfunction (moderate to severe mitral, aortic, tricuspid or pulmonary valve stenosis and/or regurgitation defined by echocardiography according to AAC/AHA Management of Valvular Heart Disease Guidelines), echocardiographic image that was technically insufficient were excluded from the study. All of the study patients were taking respective immunosuppressive medication (Table 1). Written, detailed, informed consent was obtained from all patients. The institutional ethics committee approved the study protocol. The demographic and baseline characteristics of patients in the study and control groups were given in Table 1.
CAV (+) (n=21)
CAV (-) (n=17)
p
Patient age, years
48.7 ± 9.5
43.7 ± 9.4
0.11
Donor age, years
30 ± 8
28 ± 10
0.47
Gender, male/female, (n)
15/6
10-7
0.42
Systolic blood pressure, mmHg
110 ± 15
115 ± 20
0.38
Diastolic blood pressure, mmHg
78 ± 11
80 ± 8
0.53
Mean blood pressure, mmHg
88 ± 12
91 ± 14
0,48
Heart rate, b.p.m
85 ± 16
87 ± 18
0.71
Body mass index, kg/m2
26 ± 5
24 ± 3
0.15
Diabetes, n
4
2
0.55
Hypertension, n
4
3
0.91
Smoking, n
1
2
0.44
Follow up duration, years
3.9 ± 1.8
4.2 ± 1.6
0.76
Idiopathic DCMP, n
12
15
0.76
Ischemic CMP, n
3
4
0.76
Others, n
7
7
0.76
Baseline DSA (PRA) (%)
8 ± 3
7 ± 2
0.24.
Prednisone
21
17
0.25
Cyclosporine
4
3
0.24
Tacrolimus
19
14
0.24
Azathioprine
8
7
0.24
Sirolimus or everolimus
4
3
0.25
Statin
21
17
0.24
Mycophenolate mofetil (MMF)
12
10
0.25
Anti- thymocyte globulin induction
7
4
0.25
Notes: b.p.m: beats per minute; PRA: Panel Reactive Antibody; DSA: Donor Specific Antibodies.
Table 1: Comparison of demographic and clinical properties in patients with/ without cardiac allograft vasculopathy undergoing cardiac transplantation.
Echocardiographic measurement of coronary flow reserve
All studies were recorded with a 5-12 MHz broadband linear transducer on EchoPac data storage and processing system (GE Vingmed Ultrasound, Horten, Norway) and measurements were performed off-line. Two dimensional visualization of the left anterior descending (LAD) artery was undertaken with a focal length of 40mm, lateral resolution of 0.6mm and axial resolution of 0.3mm. Doppler analysis of blood flow was evaluated at 4MHz. The sampler has been adapted to include only LAD data. Baseline spectral Doppler signals were recorded in the distal portion of the LAD. Doppler spectral tracings of flow velocity in the LAD were registered by fast Fourier transformation analysis. The spectral Doppler of the LAD flow demonstrated a characteristic biphasic flow pattern (Figure 1).
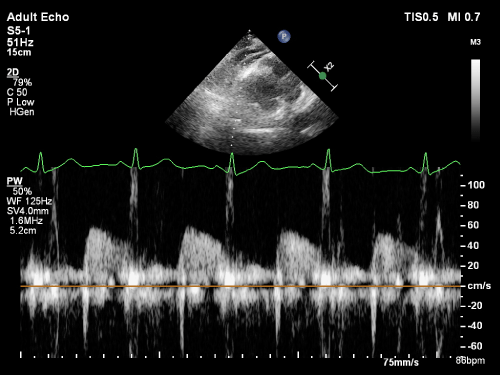
Figure 1: Measurement of diastolic and systolic flow velocity of left coronary
artery by transthoracic Doppler echocardiography.
A 6min intravenous infusion of adenosine was initiated (140mg/ kg/min) during which time hyperemic LAD flow was recorded. During the final minute of infusion, LAD diameter was again recorded in short axis to exclude further flow-induced vasodilatation. All patients had continuous heart rate and ECG monitoring. Blood pressure was recorded at baseline, every minute during adenosine infusion, and at recovery. Mean diastolic velocity (MDV) and Peak diastolic velocity (PDV) were measured at baseline and peak hyperemic conditions. An average of the measurements was obtained in three cardiac cycles. CFR was defined as the ratio of hyperemic to basal peak diastolic coronary flow velocity (CFR PDV) and the ratio of hyperemic to basal mean diastolic coronary flow velocity (CFR MDV). Normal CFR was defined as >2.0 on the basis of previous studies that evaluated flow velocities in the distal LAD [7]. The patients were divided into two groups according to respective CFR values. While CFR > 2 patients constituted cardiac allograft vasculopathy (CAV) (-) group, CFR < 2 patients were enrolled into cardiac allograft vasculopathy (CAV) (+) group.
CFR measurements were performed by two individuals who have experience in CFR measurements. In CFR measurements, intraobserver variability was assessed in 10 selected subjects at random from the patient group by repeating the measurements 3 days later under the same basal conditions. To test the interobserver variability, we performed the measurements offline from video recordings by a second observer. The intraobserver and interobserver variability for CFR calculated from 10 consecutive patients were 4.9% and 5.8% respectively.
ELISA Assay for determination of serum FGF 23-Klotho, donor specific antibodies
All blood samples were obtained from patients in the morning, after 12-hour of fasting. Samples were centrifugated at 3000rpm for 10 minutes and serum samples were aliquoted and stored at -80°C. Concentrations of human FGF 23 and klotho were analyzed by ELISA using commercial kits (Sunred Biological Technology Co. Ltd). Intra-assay and inter-assay coefficient of variations for klotho assay were <8% and <10%, respectively. The sensitivity of klotho and FGF 23 assays were 0.05ng/mL and 5.147pg/mL. Assay range of klotho was 0.1-20 ng/mL and 10-1500 pg/mL for FGF 23. 37°C incubator was used in incubation periods. Measurements were taken at 450nm using Thermo Scientific plate washer and enzyme-linked immunosorbent assay plate reader Thermo Multiscan Go (Thermo Fisher Scientific Inc). Four parameter logistic curves were used for calculating concentrations.
Panel reactive antibodies (PRA) were measured by ELISA method to estimate Anti Human Leucocyte Antibody titers. Patients were deemed appropriate for transplantation if titers were lower than 10% (PRA negative). Values greater than 10% were deemed inappropriate (PRA positive). If titers increased during follow up, Plasmapheresis was applied and biopsy intervals were shortened. 10 patients were excluded from study due to the development of AMR or CMR during follow up (6 AMR and 4 CMR). After median 4 years follow up, CFR and FGF 23-klotho analyses were done. During follow up, Coronary angiography was performed only in 12 individuals out of remaining 38 patients.
Statistical analysis
Statistical analysis was performed with SPSS software (version 20.0, SPSS Japan Inc., Tokyo, Japan). Kolmogorov-Smirnov test was used to analyze the distribution pattern of the variables. Normally distributed numerical variables were presented as mean ± standard deviation, and non-normally distributed variables were presented as median and interquartile range (IQR). Categorical variables were presented as the number and percent (%). Group means of the continuous variables were compared with Student’s t-test, Mann- Whitney U or Bonferroni corrected Mann-Whitney U tests or the Kruskal-Wallis test, where appropriate.
Student’s t-test was performed to compare the differences in the demographic, clinical, biochemical, echocardiographic parameters and the parameters such as CFR, FGF 23, klotho between the transplanted patients with/without CAV. The relationship among the parameters such as FGF 23, klotho and CFR in the transplanted patients with CAV was determined using the pearson coefficient of correlation. A p value of <0.05 was considered statistically significant with a confidence interval of 95%.
Results
There were no statistically significant difference between CAV (+) and CAV (-) groups in terms of age (48.7 ± 9.5 vs. 43.7 ± 9.4 years; p=0.11), gender (male/female 15/6 vs. 10/7; p=0.42), systolic blood pressure (110 ± 15 vs. 115 ± 20 mmHg; p=0.7), diastolic blood pressure (78 ± 11 vs. 80 ± 8 mmHg; p=0.95), heart rate (85 ± 16 vs. 87 ± 18 b.p.m; p=0.77), body mass index (26 ± 5 vs. 24 ± 3 kg/m², p=0.15), diabetes (4 (%19) vs. 2 (%12); p=0.55), smoking (1(%5), 2(%12); p=0.44). Biochemical and complete blood count parameters were also similar in both groups (Table 1).
In echocardiographic examination; LV systolic diameter, LV diastolic diameter, LV interventricular septum thickness, LV posterior wall thickness, LV ejection fraction (EF%), aortic diameter, mitral E wave, mitral A wave, deceleration time, and isovolumic relaxation time were found to be similar in both groups (Table 2). As expected, CFR values were significantly lower in patients who have CAV (1.24 ± 0.45 vs. 2.47 ± 0.27; p <0.001*)
CAV (+) (n=21)
CAV (-) (n=17)
P
LV systolic diameter, mm
29.5 ± 3.4
28.5 ± 2.7
0.15
LV diastolic diameter, mm
47.6 ± 4.5
48.4± 3.9
0.2
Interventriculer septum, mm
10.7 ± 1.2
11.1 ± 1.6
0.21
Posterior wall, mm
10.1 ± 1.6
10.4 ± 1.8
0.43
LV ejection fraction, %
60.2 ± 5.3
62.4 ± 3.5
0.39
Aortic diameter, mm
31.4± 3.5
30.8 ± 4.0
0.36
Mitral E wave, cm/s
92.23± 16.8
94.2 ± 13.4
0.45
Mitral A wave, cm/s
62.25 ± 13.1
64.42 ± 12.2
0.55
Deceleration time, ms
188.2 ± 29.3
195.3 ± 25.99
0.81
Isovolumic relaxation time, ms
62.3 ± 5.1
57.28 ± 4.3
0.38
Notes: LV: Left Ventricle; SD: Standard Deviation.
Table 2: Comparison of echocardiographic parameters in patients with/out cardiac allograft vasculopathy undergoing cardiac transplantation.
FGF values were distributed in a broader margin in CAV (+) group compared to CAV (-) group (Table 5). FGF levels were significantly higher in CAV (+) group (300.38 ± 85.03 vs. 179.88 ± 57.86; p <0.001*) (Table 3 and Figure 2). The difference between groups was independent from conventional risk factors such as hypertension and diabetes. When both groups were taken into account, There was a moderate and negative correlation between FGF levels and CFR (r=- 0.52; p=0.02). Correlation between CFR and FGF 23 levels was better when CAV (+) group analyzed separately (r=-0.71; p=0.03) (Figure 3).
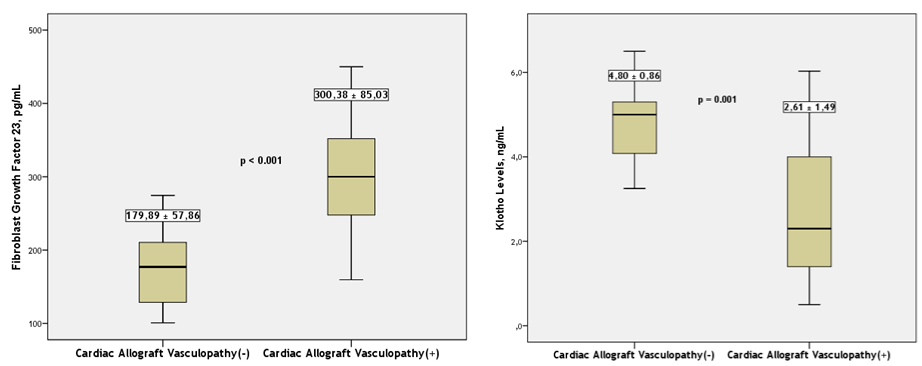
Figure 2: Distribution plot of fibroblast growth factor 23 and klotho levels in patients with/out cardiac allograft vasculopathy undergoing cardiac transplantation.
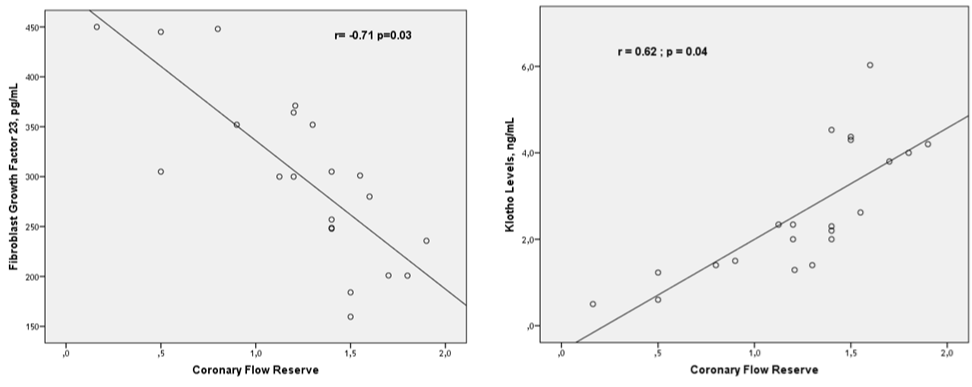
Figure 3: Correlation of coronary flow reserve with fibroblast growth factor 23 and klotho levels was better when CAV (+) group analyzed separately.
CAV (+) (n=21)
CAV (-) (n=17)
P
Coronary reserve flow
1.24 ± 0.45
2.47 ± 0.27
0.001*
FGF 23, pg/ml
300.38 ± 85.03
179.88 ± 57.86
<0.001*
Klotho, ng/ml
2.62 ± 1.49
4.81 ± 0.86
<0.001*
Table 3: Comparison of coronary flow reserve, fibroblast growth factor 23 and klotho levels in patients with/out cardiac allograft vasculopathy undergoing cardiac transplantation.
Klotho levels were significantly lower in patients who have CAV (+). Klotho levels were distributed more homogeneously compared with FGF 23 levels in patients who have CAV (+) (2.62 ± 1.49 vs. 4.81 ± 0.86; p<0.001*) (Table 3 and Figure 3). When both groups were taken into account, There was a relatively weak correlation between klotho levels and CFR (r=0.40; p=0.05). Correlation between CFR and klotho levels was better when CAV (+) group analyzed separately (r=0.62; p=0.04) (Figure 4). There was an inverse correlation between klotho and FGF 23 levels in both CAV (+) and CAV (-) groups (Figure 4).
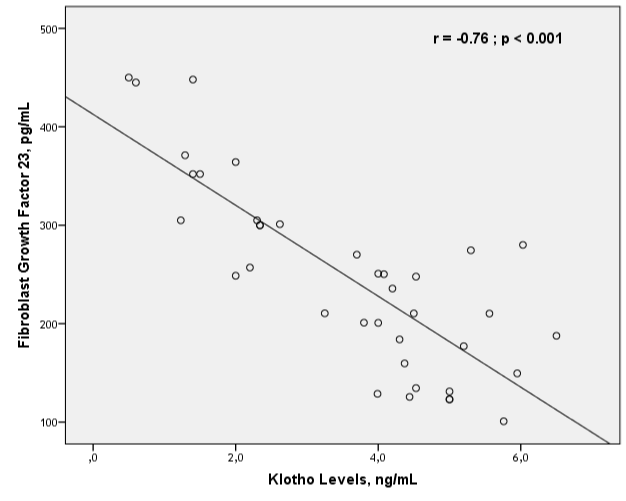
Figure 4: Correlation of fibroblast growth factor 23 and klotho levels in patients
undergoing heart transplantation regardless of allograft vasculopathy.
Coronary angiography was performed in 12 patients. 6 patients were labeled as ISHLT CAV 0, 4 of them as ISHLT CAV1-2 of them as ISHLT. None of the patients were ISHLT CAV3. All of the patients who had angiographically meaningful disease (ISHLT greater than 0) had CFR values lower than 2. Interestingly, 4 patients, out of 6 who were ISHLT O, had also CFR values lower than 2, suggesting endothelial disfunction or vasculopathy. Those 4 patients were considered to have developed CAV, even though angiography suggested otherwise. ISHLT grading and CFR values were correlated well in patients who have low CFR values CAV (+). But there were some patients who were ISHLT 0 and have CFR < 2. Those patients were considered as CAV (-) based on ISHLT CAV grading, but CAV (+) according to CFR measurement. CFR seemed to be more sensitive to detect vasculopathy compared to angiography. So, angiographic evaluation was not added to statistical calculation.
There was no relation between FGF 23-klotho levels and PRA positivity (Table 4). 8 patients were excluded from study due to the development of AMR or CMR during follow up (5 AMR and 3 CMR). 6 of them were analyzed retrospectively (Table 5). There were no significant differences in klotho and FGF 23 values in patients with and without rejection (Table 5).
CAV (+)
PRA (+)
(n=2)CAV (+)
PRA (-)
(n=19)P
CAV (-)
PRA (+)
(n=2)CAV (-)
PRA (-)
(n=15)p
CFR
1.29 ± 0.23
1.21 ± 0.18
0.24
2.35 ± 0.20
2.51 ± 0.45
0.25
FGF 23, pg/ml
289.2 ± 63.4
309.6 ± 72.4
0.69
182 ± 59.8
175 ± 48.5
0.85
Klotho, ng/ml
2.67 ± 1.23
2.58 ± 0.45
0,82
4.75 ± 0.74
4.88 ± 0.82
0.85
Table 4: Comparison of coronary flow reserve, fibroblast growth factor 23 and klotho levels according to CAV and PRA positivity.
AMR (n=4)
CMR (n=2)
No Rejection (n=38)
P value
CFR
1.79 ± 0.49
2.01 ± 0.43
1.87 ± 0.45
0.85
FGF 23, pg/ml
254 ± 72
272 ± 65
240 ± 65
0.75
Klotho, ng/ml
3.5 ± 1.35
3.7 ± 1.23
3.8 ± 1.47
0.92
Table 5: Comparison of coronary flow reserve, fibroblast growth factor 23 and klotho levels in patients with/outcardiac allograft rejection.
Discussion
The FGF family has regulatory effects on cell proliferation, migration, differentiation and survival. In chronic kidney disease (CKD) at all stages, blood levels of FGF 23 are one of the strongest known indicators of cardiovascular events and are independently and positively associated with increasing LV mass index. The relationship with LV mass index has been demonstrated in older individuals without CKD, suggesting that FGF 23 may be a cardiovascular risk factor in adults, regardless of kidney function [8,9]. FGF 23 exposure in-vitro caused direct myocardial cell hypertrophy and repetitive FGF 23 injections led to the development of LV hypertrophy. FGF 23 blood levels in humans have been linked to broader disease states of the LV, including atrial fibrillation and LV dysfunction, as well as heart failure. Serum FGF 23 levels are associated to heart failure even in early CKD, which leads to further myocardial dysfunction, potentially creating a vicious cycle. FGF 23 levels were found to be higher in patients with reduced LVEF [10].
Klotho may counteract with inflammation to protect the vascular wall integrity [11,12]. Soluble klotho suppresses tumor necrosis factor-a and adhesion molecules intercellular adhesion molecule-1 and vascular cell adhesion molecule-1 in endothelium. It was shown that endothelin-1, a harmful molecule to endothelium suppresses klotho levels [13,14]. In our study, Klotho levels displayed similar but inverse relationship with CFR compared to FGF 23 levels. Klotho levels were lower in patients who had vasculopathy and had a positive correlation with CFR levels. Klotho levels were found to be significantly higher in CAV (+) group. These results gave rise to the idea that soluble klotho had a protective effect against vasculopathy. Since there was a correlation between CFR and klotho, it was considered that klotho provides its beneficial effects by improving endothelial functions and CFR.
Elevated FGF 23 levels are independently associated with endothelial dysfunction and arterial stiffness, which have been related to cardiovascular mortality. Circulating FGF 23 levels are correlated with impaired vascular function. Due to its role in mineralization, FGF 23 was clinically associated with vascular calcification which may, in part, be caused by a deficiency of active vitamin D. Vascular klotho deficiency potentiates the development of human artery calcification and mediates resistance to FGF 23 [15-18].
Coronary microvascular function is a prognostic factor in cardiovascular disorders [19,20]. Microvascular function is reduced in coronary artery disease, even in territories without prior coronary artery stenosis and impaired microvascular function carries a poor prognosis [21,22]. CFR measured by transthoracic echocardiography with spectral Doppler measurement of coronary artery flow velocity is a non-invasive method for measurement of coronary microvascular function.
CFR is method used to evaluate the coronary macro and microvascular function. It is calculated by dividing hyperemic coronary blood flow during maximum vasodilation to resting coronary blood flow. It is a reliable, non-invasive method to identify epicardial coronary patency as well as coronary microcirculatory function. CFR represents the capacity of the coronary circulation to dilate following an increase in myocardial metabolic demands. Impaired CFR constitutes a marker of coronary microcirculatory dysfunction and reflects the impairment of the epicardial coronary artery flow in the presence of significant coronary stenosis [23,24]. In absence of significant coronary artery stenosis, CFR is considered a quantitative measure of coronary microvascular function. CFR has both diagnostic and prognostic implications and may be a useful translational tool for risk-stratification [25,26].
Pitfalls of CFR measurement by echocardiography as follows: Learning curve effect was seen in most centers in which contrast agents were not used. The flow in LAD branches could be erroneously interpreted as the flow in LAD main trunk. Images may not be sufficiently clear to allow for accurate measurement of the vessel diameter. Without estimation of the coronary artery diameter we can measure changes only in coronary blood flow velocity, but not changes in coronary blood flow. However, both parameters are closely correlated and even in large invasive studies DEBATE and DESTINI, the CFR derived from changes only in the velocity of coronary blood flow was accepted instead of the absolute coronary blood flow, which is measurable during invasive studies. CFR by Doppler provided a highly satisfying correlation between non-invasive and invasive measurements [27,28].
ISHLT CAV classification was develop to stratify the degree of allograft vasculopathy. This measure divides patients to four categories according to angiographic findings. ISHLT CAV O (Not significant, No detectable angiographic lesion), ISHLT CAV1 (Mild, Angiographic left main (LM) <50%, or primary vessel with maximum lesion of <70%, or any branch stenosis <70% (including diffuse narrowing) without allograft dysfunction), ISHLT CAV2 (Moderate, Angiographic LM <50%; a single primary vessel >70%, or isolated branch stenosis >70% in branches of 2 systems, without allograft dysfunction. ISHLT CAV3 (Severe, Angiographic LM >50%, or two or more primary vessels >70% stenosis, or isolated branch stenosis >70% in all 3 systems) [29].
ISHLT CAV classification marks the lesion as grade 1 even if they become as apparent as 69% (below 70%). ISHLT CAV grade 0 labels coronary arteries as normal and fails to detect any smoldering endothelial pathology or underlying dysfunctional response. As a matter of fact, coronary angiography is not gold standard to detect CAV and endothelial dysfunction especially in early phases. Coronary angiography can detect lesions only after they protrude towards vessel lumen. Taking into account the nature of diffuse narrowing of vessels in CAV, sometimes it is difficult to interpret angiography in early stages. Additionally, Angiography may not elicit information about coronary reserve or endothelial function without additional tests (i.e. intracoronary adenosine, achetylcoline or papaverine). In summary, Angiography only provides information after the disease has become evident. As CFR studies conducted by echocardiography could exhibit endothelial disease before the angiographically evident changes occur, this type of study design is more suitable to serve our purpose. Our study aimed at the ethiopathology of the disease and it was important to detect the disease at early stages. CFR measured by echocardiography was superior to angiographic evaluation in our study design. Accordingly, Coronary angiography was not an essential part of the study. If intravascular ultrasonography had been performed, It would be valuable to detect early disease but it would be again impossible to perform physiologic evaluation. CFR with invasive methods could have been appropriate but performing invasive procedure without clear indication could have led to ethical considerations.
All of the patients who had angiographically meaningful disease (ISHLT greater than 0) had CFR values lower than 2. Interestingly, 4 patients, out of 6 who were ISHLT 0, had also CFR values lower than 2, suggesting endothelial disfunction or vasculopathy. Those 4 patients were considered to have developed CAV, even though angiography suggested otherwise. The fact that some patients with CFR < 2 had ISHLT > 0 was estimated as an important finding to show the reliability and sensitivity of our method. Therefore, Coronary flow reserve measurement was chosen to detect coronary artery disease and to delineate the relationship between FGF 23 - klotho levels and allograft vasculopathy. While CFR > 2 patients constituted cardiac allograft vasculopathy (CAV) (-) group, CFR < 2 patients were defined as CAV (+) group.
The detection of high FGF 23 levels in CAV (+) group and good correlation between FGF 23 and CVR values suggests that FGF 23 levels decrease in accordance with the severity of the coronary disease. As the differences between groups were not associated with conventional cardiovascular risk factors, it was considered that there was an inverse relation between FGF 23 levels and allograft vasculopathy. This finding suggests that besides FGF 23 becomes increasingly known risk factor for atherosclerosis. It may also have a role in the development of CAV. Allograft vasculopathy is generally considered as a partly different entity compared to atherosclerosis. While atherosclerosis generates more localized and lipid rich lesions, Allograft vasculopathy tend to be more extensive along the coronary vasculature. Immunosuppressive medication given to transplanted patients and tissue mismatch were also held responsible for the allograft vasculopathy [30]. But in the common sense, endothelial dysfunction is responsible for the beginning and progression of both atherosclerotic disease and allograft vasculopathy. There are clinical associations between elevated FGF 23 and atherosclerosis, impaired flow-mediated dilation, impaired vasoreactivity, and arterial stiffness [31-33].
The imbalance is mainly caused by reduced nitric oxide bioavailability and/or increased generation of reactive oxygen species. In a study, FGF 23 levels were significantly correlated with multivessel disease [34]. Higher levels of FGF in vasculopathy group and inverse relationship with coronary flow reserve indicate that FGF 23 may have a role in the pathogenesis of cardiac allograft vasculopathy.
Our study couldn’t find any relation between FGF 23-klotho levels and PRA positivity and rejection (Table 4 and 5). Vasculopahy seems to be a process of disease independent from PRA and rejection on account of FGF 23-klotho system.
Conclusion
In heart transplantation patients, FGF 23 levels were found to be significantly higher in patients who had CAV (+) compared to patients who had normal CFR. There was a good but negative correlation between CFR and FGF 23 levels. Conversely, Klotho levels were significantly lower in vasculopathy group. There was a good correlation between CFR and klotho levels. These results gave rise to the thought FGF 23-klotho axis has a role in the development of allograft vasculopathy.
Limitations
CAV was diagnosed by coronary flow reserve measurement. Coronary angiography assisted intravascular ultrasonography or optical coherent tomography might be very useful to detect anatomical changes in addition to physiologic changes examined by CFR. Echo images sometimes might not be satisfactory enough to allow for accurate measurement of the vessel diameter and calculations were limited to measurements of coronary blood flow velocity. Learning curve effect was seen in most centers in which contrast agents were not used. Additionally, The flow in LAD branches could be erroneously interpreted as the flow in LAD main trunk. Study was designed to explore the effect of FGF 23-klotho on CAV. The number of patients who have rejection and/or PRA positivity may not be sufficient to measure the effect of FGF 23-klotho on rejection.
References
- Recio-Mayoral A, Banerjee D, Streather C, Kaski JC. Endothelial dysfunction, inflammation and atherosclerosis in chronic kidney disease a crosssectional study of predialysis, dialysis and kidney-transplantation patients. Atherosclerosis. 2011; 216: 446-451.
- Montezano AC, Touyz RM. Reactive oxygen species and endothelial function-role of nitric oxide synthase uncoupling and Nox family nicotinamide adenine dinucleotide phosphate oxidases. Basic Clin Pharmacol Toxicol. 2012; 110: 87-94.
- Yamamoto M, Clark JD, Pastor JV, et al. Regulation of oxidative stress by the anti-aging hormone klotho. J Biol Chem. 2005; 280: 38029-38034.
- Bartke A. Long-lived Klotho mice: new insights into the roles of IGF-1 and insulin in aging. Trends Endocrinol Metab. 2006; 17: 33-35.
- White KE, Evans WE, Speer MC, et al. Autosomal dominant hypophosphateamic ricketsis associated with mutations in FGF23. Nat Genet. 2000; 26: 345-348.
- Arking DE, Krebsova A, Macek M Sr., et al. Association of human aging with a functional variant of klotho. Proc Natl Acad Sci USA. 2002; 99: 856-861.
- Drexler H, Zeiher AM, Wollschlager H, Meinertz T, Just H, Bonzel T. Flowdependent coronary artery dilatation in humans. Circulation. 1989; 80: 466- 474.
- Lim K, Lu TS, Molostvov G, Lee C, Lam FT, et al. Vascular Klotho deficiency potentiates the development of human artery calcification and mediates resistance to fibroblast growth factor 23. Circulation. 2012; 125: 2243-2255.
- Fliser D, Kollerits B, Neyer U, Ankerst DP, Lhotta K, Lingenhel A, et al. Fibroblast growth factor 23(FGF23) predicts progression of chronic kidney disease: the Mild to Moderate Kidney Disease (MMKD) Study. J Am Soc Nephrol. 2007: 2600-2608.
- Miki Imazu, Hiroyuki Takahama, Hiroshi Asanuma, et al. Pathophysiological impact of serum fibroblast growth factor 23 in patients with nonischemic cardiac disease and early chronic kidney disease. Am J Physiol Heart Circ Physiol. 2014; 307: H1504-H1511.
- Six I, Okazaki H, Gross P, et al. Direct, acute effects of klotho and FGF23 on vascular smooth muscle and endothelium. PLoS One. 2014; 9: e93423.
- Saito Y, Yamagishi T, Nakamura T, et al. Klotho protein protects against endothelial dysfunction. Biochem Biophys Res Commun. 1998; 248: 324- 329.
- Zuo Z, Lei H, Wang X, et al. Aging-related kidney damage is associated with a decrease in klotho expression and an increase in superoxide production. Aging (Dordr). 2011; 33: 261-274.
- Wang YH, Sun ZJ. Antiaging gene klotho regulates endothelin- 1 levels and endothelin receptor subtype B expression in kidneys of spontaneously hypertensive rates. J Hypertens. 2014; 32: 1629-1636.
- Coen G, De Paolis P, Ballanti P, Pierantozzi A, Pisano S, Sardella D, et al. Peripheral artery calcifications evaluated by histology correlate to those detected by CT: relationships with fetuin-A and FGF-23. J Nephrol. 2011; 24: 313-321.
- Ishimura E, Nishizawa Y. Role of fibroblast growth factor-23 in peripheral vascular calcification in non-diabetic and diabetic hemodialysis patients. Osteoporos Int. 2006; 17: 1506-1513.
- Nasrallah MM, El-Shehaby AR, Salem MM, Osman NA, El Sheikh E, Sharaf El Din UA. Fibroblast growth factor-23 (FGF-23) is independently correlated to aortic calcification in haemodialysis patients. Nephrol Dial Transplant. 2010; 25: 2679-2685.
- Schoppet M, Hofbauer LC, Brinskelle-Schmal N, Varennes A, Goudable J, Richard M, et al. Serum level of the phosphaturic factor FGF23 is associated with abdominal aortic calcification in men: the STRAMBO study. J Clin Endocrinol Metab. 2012; 97: E575-583.
- Crea F, Camici PG, Bairey Merz CN. Coronary microvascular dysfunction: an update. Eur Heart J. 2014; 35: 1101-1111.
- Taqueti VR, Hachamovitch R, Murthy VL, Naya M, Foster CR, Hainer J, et al. Global coronary flow reserve is associated with adverse cardiovascular events independently of luminal angiographic severity and modifies the effect of early revascularization. Circulation. 2014; 1-9.
- Uren NG, Marraccini P, Gistri R, de Silva R, Camici PG. Altered coronary vasodilator reserve and metabolism in myocardium subtended by normal arteries in patients with coronary artery disease. J Am Coll Cardiol. 1993; 22: 650-658.
- Cortigiani L, Rigo F, Gherardi S, Bovenzi F, Picano E, Sicari R. Implication of the continuous prognostic spectrum of Doppler echocardiographic derived coronary flow reserve on left anterior descending artery. Am J Cardiol. 2010; 105: 158-162.
- Cortigiani L, Rigo F, Gherardi S, Bovenzi F, Picano E, Sicari R. Implication of the continuous prognostic spectrum of Doppler echocardiographic derived coronary flow reserve on left anterior descending artery. Am J Cardiol. 2010; 105: 158-162.
- Ikonomidis I, Makavos G, Lekakis J. Arterial stiffness and coronary artery disease. Curr Opin Cardiol. 2015; 30: 422-431.
- Olsen RH, Pedersen LR, Jürs A, Snoer M, Haugaard SB, Prescott E. A randomized trial comparing the effect of exercise training and weight loss on microvascular function in coronary artery disease. Int J Cardiol. 2015; 185: 229-235.
- Ruscazio M, Montisci R, Bezante G, Caiati C, Balbi M, Tona F, et al. Early noninvasive evaluation of coronary flow reserve after angioplasty in the left anterior descending coronary artery identifies patients at high risk of restenosis at follow-up. J Am Soc Echocardiogr. 2012; 25: 902-910.
- Di Mario C, Moses JW, Anderson TJ, et al. Randomized comparison of elective stent implantation and coronary balloon angioplasty guided by online quantitative angiography and intracoronary Doppler. DESTINI Study Group (Doppler Endpoint Stenting International Investigation). Circulation. 2000; 102: 2938-2944.
- Serruys PW, de Bruyne B, Carlier S, et al. Randomized comparison of primary stenting and provisional balloon angioplasty guided by flow velocity measurement. Doppler Endpoints Balloon Angioplasty Trial Europe (DEBATE) II Study Group. Circulation. 2000; 102: 2930-2937.
- Mandeep R Mehra, Maria G Crespo-Leiro, Anne Dipchand, et al. International Society for Heart and Lung Transplantation working formulation of a standardized nomenclature for cardiac allograft vasculopathy. The Journal of Heart and Lung Transplantation. 2010; 29: 717-727.
- Maziar Rahmani, Rani P Cruz, David J. Allograft Vasculopathy Versus Atherosclerosis. Granville, Bruce M. McManus Circ Res. 2006; 99: 801-815.
- Mirza MA, Hansen T, Johansson L, Ahlstrom H, Larsson A, Lind L, et al. Relationship between circulating FGF23 and total body atherosclerosis in the community. Nephrol Dial Transplant. 2009; 24: 3125-3131.
- Mirza MA, Larsson A, Lind L, Larsson TE. Circulating fibroblast growth factor-23 is associated with vascular dysfunction in the community. Atherosclerosis 2009; 205: 385-390.
- Yilmaz MI, Sonmez A, Saglam M, Yaman H, Kilic S, Demirkaya E, et al. FGF- 23 and vascular dysfunction in patients with stage 3 and 4 chronic kidney disease. Kidney Int. 2010; 78: 679-685.
- Hu X, Ma X, Pan X, et al. Fibroblast growth factor 23 is associated with the presence of coronary artery disease and the number of stenotic vessels. Clin. Ecp. Pharmacol. 2015; 42: 1152-1157.