
Research Article
Austin J Clin Cardiolog. 2022; 8(1): 1089.
Efficacy of Evolocumab Therapy in Patients with Acute Coronary Syndrome in the Very Early Phase for Reducing Plaque Vulnerability Assessed by Optical Coherence Tomography
Yano H¹*, Horinaka S², Fukushi T¹, Miyaishi Y¹, Kuribara J¹, Kawaguchi R¹ and Naito S¹
¹Department of Cardiology, Gunma Prefectural Cardiovascular Center, Maebashi, Gunma, Japan
²Department of Cardiology and Nephrology, Dokkyo Medical University, Mibu, Tochigi, Japan
*Corresponding author: Hideki Yano, Department of Cardiology, Gunma Prefectural Cardiovascular Center, Maebashi, Gunma, 371-0004, Japan
Received: February 23, 2022; Accepted: March 15, 2022; Published: March 22, 2022
Abstract
Background: Proprotein convertase subtilisin/kexin type 9 inhibitor, evolocumab, has been demonstrated to produce significantly greater reduction in LDL cholesterol levels and cardiovascular events than standard statin therapy in patients with coronary artery disease. Whereas, effect on fibrouscup thickness or extension of the atherosclerotic plaque with early therapy with PCSK9-inhibitor within 1-week after onset of Acute Coronary Syndrome (ACS) has never been reported.
Methods: Patients were non-randomly allocated to either the early evolocumab group (received evolocumab 140 mg every 2 weeks within 1-week after onset of ACS) or the late evolocumab group (from 4-week after onset of ACS). Optical Coherence Tomography (OCT) was performed to assess intermediate, non-culprit lesions just 4 and 36 weeks after emergent percutaneous coronary intervention.
Results: The decrease in Low-Density Lipoprotein Cholesterol (LDL-C) was greater in the early than in the late group between baseline and 4-week followup (reduction rate: -69.4% vs. -32.5%). However, the percentage of decrease in LDL-C was comparable in the two groups between baseline and 36-week follow-up. OCT analysis revealed that the increase in fibrous-cap thickness was greater in the early than in the late group between baseline and 4-week followup (early evolocumabgroup: +32μm, late evolocumab group: +18um). However, the percentage of increase in fibrous-cap thickness increased comparably in the 2 groups between baseline and 36-week follow-up.
Conclusions: Evolocumab therapy in the very early phase produced incremental growth in fibrous-cap thickness, which was associated with greater reduction of LDL-C even in the short term in the early phase of ACS compared to the late evolocumab group.
Keywords: Evolocumab; Optical coherence tomography; Fibrous cap thickness; Plaque stabilization
Abbreviations
LDL-C: Low-Density Lipoprotein Cholesterol; ACS: Acute Coronary Syndrome; IVUS: Intravascular Ultrasound; OCT: Optical Coherence Tomography; PCSK9: Proprotein Convertase Subtilisin/ Kexin Type 9; PCI: Percutaneously Coronary Intervention; STEMI: ST-segment Elevation Myocardial Infarction; NSTEMI: Non-STSegment Elevation Myocardial Infarction; TCFA: Thin-Cap Fibro Atheroma
Introduction
Several large-scale, multicenter, randomized trials have shown the importance of Low-Density Lipoprotein Cholesterol (LDL-C) regulation with 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors, statins, on the risk of cardiovascular events or death [1,2].
Previous clinical trials have shown that although patients experience the highest risk of death and recurrent is chaemic events in the early Post-Acute Coronary Syndrome (ACS) period, these early events can be reduced by early onset of statin therapy [3,4].
The imaging studies using grayscale Intravascular Ultrasound (IVUS) have reported that statin therapy results in major suppression [5], or even regression, of Atheroma volume in the atherosclerotic coronary arteries [6]. However the grayscale IVUS is appropriate for plaque volume evaluation, it does not have the spatial resolution to accurately measure the thickness of the fibrous cap. Unlike other imaging modalities, intravascular Optical Coherence Tomography (OCT) is a high-resolution imaging technique for plaque characterization. OCT can evaluate the measurement of fibrouscap thickness, thought to be a major factor in plaque vulnerability [7]. Previous study demonstrated that more potent lipid-lowering therapy by statin in patients induces significant plaque regression and, by decreasing plaque lipid content and increasing plaque fibrous cap thickness, induces plaque stabilization [8]. Besides, the fibrouscap thickness was demonstrated to be increased by the aggressive ipid-lowering therapy with statin after acute myocardial infarction [9]. However, in a high CV risk population in a routine care setting in Japan, a previous study reported that guideline recommended LDL-C goal attainment was low. In addition, physicians should emphasize the need for more potent lipid-lowering therapy in this population [10].
Therefore, a beneficial increase in fibrous-cap thickness after statin treatment may be established to translate into substantial decreases in clinical events as a benchmark for future investigational agents that target plaque instability.
Then evolocumab, a fully human monoclonal antibody that inhibits Proprotein Convertase Subtilisin/Kexin Type 9 (PCSK9) from binding to LDL receptors on the liver surface, significantly have emerged as a novel treatment option for effectively lowering LDL-C levels by approximately 60% [11]. Further research data reported that PCSK9 might also accelerate atherosclerosis by promoting inflammation, endothelial dysfunction, and hypertension by mechanisms beyond degradation of hepatic LDL receptors [12,13].
In the Global Assessment of plaque regression with a PCSK9 antibody as measured by an intravascular ultrasound (GLAGOV) trial, among patients with angiographic coronary disease treated with statins, addition of evolocumab, compared with placebo, resulted in a greater decrease in percent atheroma volume [14]. Additionally, recent randomized multicenter trials clearly demonstrated that use of a PCSK9 inhibitor was associated with a significantly reduced incidence of adverse clinical events in patients with high-risk stable coronary artery disease [15] or ACS [16]. Recently, we reported that adding the PCSK9-inhibitor evolocumab to statin therapy might produce incremental growth in fibrous cap thickness and regression of the lipid-rich plaque after ACS [17]. However, as mentioned above, the risk of death and recurrent ischaemic events in ACS patients is highest in the early post-onset period. Then it is still unclear whether the timing of the introduction of evolocumab will have an effect on plaque regression. In particular, the effect of very early PCSK9- inhibitor evolocumab therapy on plaque vulnerability in patients with ACS remains unknown compared to the late evolocumab group. Because of that, the aim of the present study was to assess the effect of very early PCSK9 inhibitor evolocumab therapy on fibrous-cap thickness in coronary atherosclerotic plaques of patients with ACS by using OCT.
Materials and Methods
Study patients and design
This study was a retrospective, non-randomized, observational, two centers (Dokkyo Medical University Hospital and Gunma Prefectural Cardiovascular Center) study. From March 2017 to 2021 Apr, June, all consecutive patients with multivessel disease who had untreated dyslipidemia (defined as serum LDL-C level >100mg/dL) and received emergent Percutaneously Coronary Intervention (PCI) after ACS and OCT imaging were evaluated. Of these, data from 56 patients using OCT to compare the effect of PCSK9 inhibitor evolocumab therapy on fibrous-cap thickness in coronary atherosclerotic plaque between an early evolocumaband late evolocumab groups were extracted. All patients were treated with rosuvastatin 5mg once daily from baseline (within 24h after the OCT examination) for aggressive lipid-lowering therapy, which is a secondary prevention of ACS. Patients in the early evolocumab group received evolocumab (140mg every 2 weeks) within 1 week after onset of ACS, whereas patients in the late evolocumab group received evolocumab (140mg every 2 weeks) from 4 weeks after baseline.
After receiving informed consent, we performed staged PCI for the residual lesion 4 weeks after emergent PCI and conducted follow-up coronary angiography 36 weeks thereafter. The follow-up target lesions of the OCT analysis were de novo, intermediate, and nonculpritcoronary lesion in patients with ACS. Five patients were excluded (one patient refused, and four patients failed OCT analysis), and the remaining 51 patients were fully examined in this study (Figure 1).
In this study, ACS is defined as ST-Segment Elevation Myocardialinfarction (STEMI), Non-ST-Segment Elevation Myocardial Infarction (NSTEMI), or unstable angina. The follow-up target lesion of the OCT analysis had a diameter stenos is percentage of 30% - 70% by visual estimation on angiogram. If more than two de novo, intermediate, or non-culprit lesions were recognized, the most severely stenotic lesion was selected as the target lesion in the OCT analysis. The target lesion could be in both the PCI-treated and non-PCI-treated coronary arteries, where the target lesion was >10mm apart. Exclusion criteria included left main trunk lesions, cardiogenic shock, recommended coronary artery bypass grafting, severechronic kidney disease, unsuccessful PCI, and current use of any lipid-lowering therapy.
OCT image protocol and analysis
OCT was performed using the ILUMIEN OCT imaging system (Abbott Vascular, Santa Clara, CA, USA) with a motorized pullback system at 20mm/s and a rotation speed of 100frames/s, using a non-occlusive technique. The OCT images were digitally stored for offline analysis. The OCT images were obtained and reviewed side by side at baseline, 4-week follow-up, and 36-week follow-up. Target lesions between baseline and follow-up OCT were matched based on their distances from landmarks, such as branches and calcifications. Independent, experienced OCT investigators, blinded to the patient groups, measured fibrous-cap thickness using a dedicated offline review system (St. Jude Medical Inc., St. Paul, MN, USA) at the laboratory. The calibration was adjusted before the OCT analysis. The minimum lumen area in each target lesion was determined using an automated measurement algorithm and additional manual corrections. The plaque tissue was characterized using previously validated criteria [18]. The fibrous cap was identified as a lesion with high back scattering and a relatively homogeneous OCT signal. The lipid or necrotic core was identified as a signal-poor region with poorly delineated borders, little or no signal backscattering, and an overlying signal-rich layer, the fibrous cap. The minimum fibrous cap thickness was calculated using a previously reported method [19]. In brief, the fibrous cap thickness of each lipid-rich plaque was measured, first at 1mm intervals over the lipid plaque then three times at its thinnest part at each cross-section, and the average value was calculated. Minimum fibrous cap thickness was determined as the smallest fibrous cap thickness in the three candidate frames selected by manual screening (Figure 2). The maximum lipid arc was defined as the largest lipid arc from the center of the lumen in the three candidate frames selected by visual screening (Figure 2). Although the fluctuation by the OCT catheter position could be influenced by the largest lipid arc, a frame was selected to be as similar as possible to the side branch and the lesion morphology, and the center of the lumen was determined to measure the largest lipid arc. Lipid length was calculated from the number of frames with lipid cores.
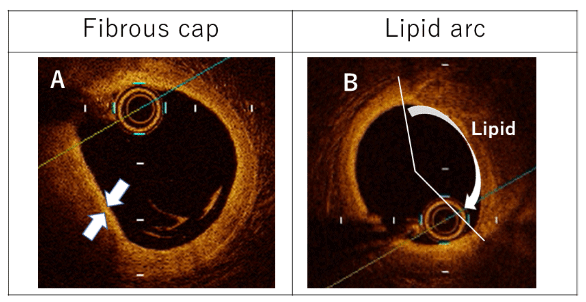
Figure 2: Representative optical coherence tomography images of (A) fibrous cap and (B) lipid arc.
We also performed macrophage semi-quantification on the same OCT cross-sections used for qualitative plaque assessment, according to the OCT macrophage grading system, to semi-quantify the bright spots based on axial and circumferential distribution, as follows: Grade 0, no macrophage; grade 1, localized macrophage accumulation; grade 2, clustered accumulation <1 quadrant; grade 3, clustered accumulation ≥1 quadrant and <3 quadrants; and grade 4, clustered accumulation ≥3 [20] as shown in (Figure S1).
The study complied with the ethical principles of the Helsinki Declaration.
Statistical analysis
All calculated data are expressed as mean ± SD. One-way repeated analysis of variance, which was subsequently subjected to a posthoc analysis (Scheffe’s test) for multiple comparisons, was used to determine the statistical significance of differences. The chi-squared test was used to analyze categorical variables with percentages. Statistical analysis was conducted using a commercially available statistical software program (JMP 13, SAS Institute, Cary, NC, USA). Statistical significance was set at p< 0.05.
Results
Baseline characteristics
51 patients were classified into two groups according to the early evolocumab group (n=25) or the late evolocumab group (n=26), as shown in (Figure 1 and Table 1). Contains patient and angiographic characteristics, medication, procedural, and lesion characteristics. The 2 groups were well matched at baseline, and their pattern of use of concomitant medications was comparable. Approximately 65% of them were STEMI cases, and all the participants were prescribed antiplatelet therapy just before PCI. At baseline, the serum total cholesterol, LDL-C, high-density lipoprotein cholesterol, triglyceride, and hemoglobin A1c did not differ between the groups (Table 2). At week 4 and 36, the serum LDL cholesterol levels were significantly decreased in both groups compared with baseline. At the 4-week follow-up, the percentage of decrease in serum LDL-C was significantly greater (reduction rate: -67.0% vs. -32.9%, respectively, p<0.001) and serum LDL-C level was significantly lower (39.8mg/dl vs. 82.6mg/dl, p<0.001) in the early evolocumab group as opposed to the late evolocumab group (Figure 3 and Table 2). The serum LDL-C level at 36-week follow-up and the percentage of decrease in serum LDL-C levels between baseline and 36-week follow-up were similar in the 2 groups (reduction rate: -74.8% vs. -74.5%, respectively, p=0.740) (Figure 3 and Table 2).
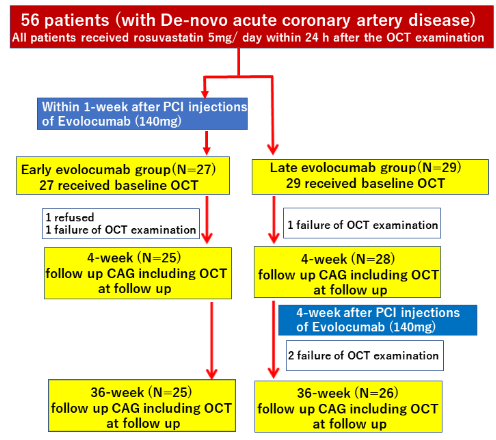
Figure 1: Study flowchart.
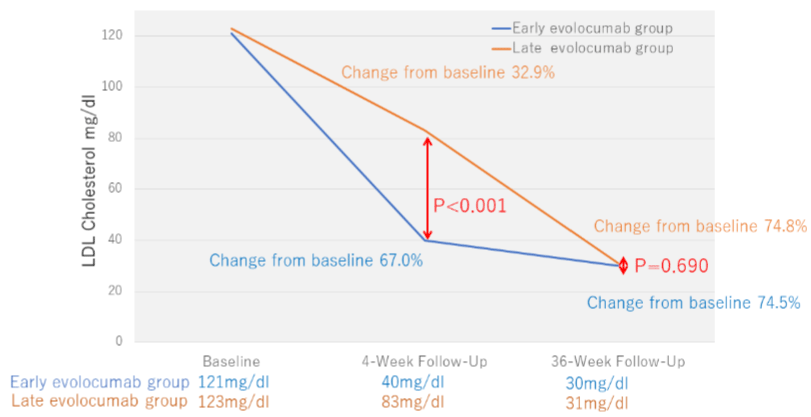
Figure 3: Low-Density Lipoprotein (LDL) cholesterol levels over time.
The absolute and percentage reductions in LDL cholesterol level in the early evolocumab group are compared to those in the late evolocumabgroup.
P value
Age, year
64.2 ± 6.4
65.1 ± 5.0
0.198
Male
19 (76.0%)
20 (76.9%)
0.551
Body Mass Index, (kg/m2)
24.4 ± 4.0
24.2 ± 4.6
0.678
Hypertension, (%)
18 (72.0%)
19 (73.1%)
0.407
Diabetes mellitus, (%)
12 (48.0%)
13 (50.0%)
0.361
Estimated Glomerular Filtration Rate (GFR) <60, (%)
10 (40.0%)
9 (34.6%)
0.194
Current smoker, (%)
12 (48.0%)
11 (42.3%)
0.308
Ejection fraction, (%)
49.8 ± 10.1
50.4 ± 7.8
0.243
STEMI / NSTEMI / uAP (n)
16/4/2005
17/04/2005
0.643
The medications just before PCI
ACEI/ARB (n)
15 (60.0%)
16 (61.5%)
0.147
Calcium channel blockers (n)
8 (32.0%)
8 (30.8%)
0.169
Beta blockers (n)
3 (12.0%)
4 (15.4%)
0.128
Nicolandil (n)
2 (8.0%)
2 (7.7%)
0.654
DAPT (n)
25 (100%)
26 (100%)
1
Target lesion
LMT
0
0
-
LAD
10 (40.0%)
10 (38.4%)
0.452
LCX
4 (16.0%)
3 (11.5%)
0.18
RCA
11 (44.0%)
13 (50.0%)
0.21
Location of target plaque
Culprit vessel
4 (16.0%)
3 (11.5%)
0.322
Non-culprit vessel
21 (84.0%)
23 (88.5%)
0.401
Mean reference diameter (mm)
2.77 ± 0.26
2.73 ± 0.30
0.293
Lesion length (mm)
13.39 ± 3.26
12.77 ± 4.2
0.256
Minimum lumen diameter (mm)
1.61 ± 0.27
1.65 ± 0.33
0.203
Percent stenosis diameter (%)
46.64 ± 13.02
47.99 ± 13.37
0.409
Data are expressed as numbers (%) or mean ± SD.
STEMI: ST-Elevation Myocardial Infarction; NSTEMI: Non-ST Elevation Myocardial Infarction; uAP: Unstable Angina Pectoris; ACEI: Angiotensin-Converting Enzyme Inhibitor; ARB: Angiotensin Receptor Blocker; DAPT: Dual Antiplatelet Therapy; RCA: Right Coronary Artery; LAD: Left Anterior Descending Artery; LCx: Left Circumflex Artery; ACC/AHA: American College of Cardiology/ American Heart Association.
Table 1: Baseline patient and lesion characteristics in the statin monotherapy and evolocumab groups.
Baseline
4-week follow-up
36-week follow-up
Early evolocumab group N = 25
Late evolocumab group N = 26
P value
Early evolocumab group N = 25
P value
Early evolocumab group N = 25
Late evolocumab group N = 26
P value
TC, mg/dL
193.6 ± 30.8
197.5 ± 33.0
0.504
124.4 ± 22.5*
156.0 ± 26.3*
<0.001
115.0 ± 23.0*
112.7 ± 24.8*
0.799
LDL-C, mg/dL
120.6 ± 20.7
123.1 ± 21.8
0.606
39.8 ± 12.5*
82.6 ± 14.6*
<0.001
30.4 ± 12.8*
31.3 ± 11.1*
0.69
HDL-C, mg/dL
44.0 ± 13.7
46.1 ± 14.4
0.187
47.6 ± 13.6
42.1 ± 14.3*
0.091
49.7 ± 13.8*
49.9 ± 14.0*
0.308
TG, mg/dL
107.3 ± 40.3
109.8 ± 43.4
0.609
104.3 ± 43.9
105.7 ± 42.9
0.714
100.1 ± 47.8
102.8 ± 46.9
0.67
HbA1c, %
6.0 ± 0.4
6.1 ± 0.5
0.705
5.8 ± 0.3
6.0 ± 0.6
0.505
5.7 ± 0.7
5.9 ± 0.8
0.54
*P< 0.05 vs. baseline.
TC: Total Cholesterol; TG: Triglyceride; HDL-C: High-Density Lipoprotein Cholesterol; LDL-C: Low-Density Lipoprotein Cholesterol; HbA1c: Hemoglobin A1c.
Table 2: Blood Sample Data.
Serial change of OCT findings
Table 3 summarizes the OCT measurements. Figure 4 shows the percentage of changes in OCT measurements during follow-up. The minimum fibrous cap thickness, maximum lipid arc, lipid length, and macrophage grade did not differ between the groups at baseline. The minimum fibrous cap thickness in both groups significantly increased from baseline to the 4-week follow-up (early evolocumab group: +32μm, late evolocumab group: +18um), and to 36-week follow-up (earalyevolocumab group: +73um, late evolocumab group: +77um). The percentage of increase in fibrous cap thickness was significantly greater in the earalyevolocumab group between baseline and 4-week follow-up (Figure 4a). However, the percentage of increase in minimum fibrous-cap thickness between baseline and 36-week follow-up was similar in the two groups, as shown in Figure 4b. The maximum lipid arc and macrophage grade in both groups significantly reduced from baseline to 4-week-or 36-week follow-up (lipid arc; early evolocumab group: -32°, late evolocumab group: -34°, macrophage grade: Early evolocumab group: -5.2, late evolocumab group, -4.9). The percentage change in the maximum lipid arc and macrophage grade between baseline and 4-week follow-up was significantly greater in the early evolocumab group than in the late evolocumab group (Figure 4a). However, the percentage of decrease in the maximum lipid arc and macrophage grade between baseline and 36-week follow-up was similar in the two groups, as shown in (Figure 4b).
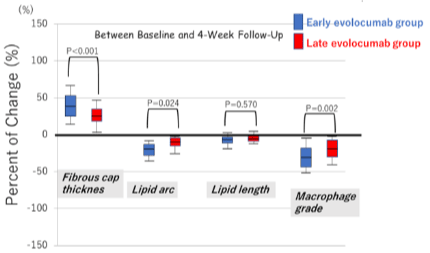
Figure 4a: Percentage of change in optical coherence tomography
measurements between baseline and 4-week follow-up. Between baseline
and 4-week follow-up.
Percentages of change in fibrous cap thickness, lipid arc, and macrophage
grade were significantly greater in the early evolocumab group than in the
late evolocumab. The percentage of change in lipid length was similar in both
groups.
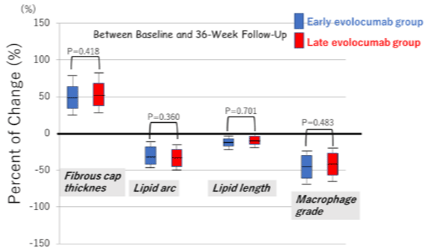
Figure 4b: The percentage of change in minimum fibrous-cap thickness,
lipid arc, macrophage grade, and lipid length between baseline and 36-week
follow-up.
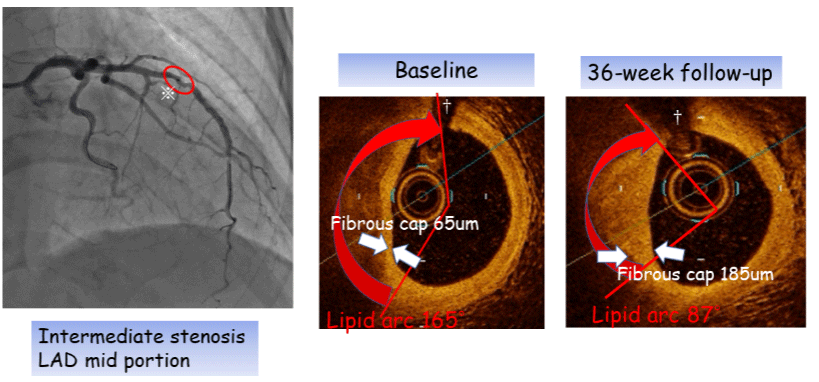
Figure 5: Representative OCT imaging in the early evolocumab group.
Fibrous-cap thickness (white arrows) increased between baseline (65 μm) and 36-week follow-up (185 μm).
Lipid arc (red arrows) decreased during the follow-up period.
※Intermediate stenos is (mid portion of the left anterior descending artery), †Side branch.
OCT: Optical Coherence Tomography.
Baseline
4-week follow-up
36-week follow-up
Early evolocumab group N = 25
Late evolocumab group N = 26
P value
Early evolocumab group N = 25
Late evolocumab group N = 26
P value
Early evolocumab group N = 25
Late evolocumab group N = 26
P value
Minimum fibrous-cap thickness, μm
122.8 ± 31.0
120.9 ± 37.6
0.44
155.0± 31.7*
139.1± 29.3*
0.01
195.5 ± 33.8*+
197.7 ± 31.7*+
0.256
Lipid arc, degree
130.4 ± 35.7
134.5 ± 40.5
0.273
115.0 ± 40.9*
126.0 ± 37.2*
0.006
98.8 ± 39.7*+
100.1 ± 36.3*+
0.543
Lipid length, mm
8.8 ± 2.5
8.6 ± 2.7
0.605
8.3 ± 2.3
8.4 ± 2.1
0.243
8.2 ± 2.3*
8.1 ± 2.0*
0.618
Macrophage grade
12.2 ± 2.3
12.0 ± 2.5
0.618
9.1 ± 2.0*
10.2 ± 2.4*
0.022
7.0 ± 2.3*+
7.1 ± 1.9*+
0.412
*P < 0.05 vs. baseline. +P < 0.05 vs. 4-week follow-up.
Table 3: OCT Measurements.
Major adverse cardiac events
No patients experienced major adverse cardiac events, such as target vessel revascularization, cardiac death, nonfatal myocardial infarction, and ST elevation myocardial infarction/non-ST elevation myocardial infarction for 36 weeks after primary PCI or during OCT examination in this study.
Discussion
The principal findings of the present study were as follows: 1) between baseline and 4-week follow-up, the percentage of decrease in serum LDL-C was significantly greater and serum LDL-C level was significantly lower in the early evolocumab group than in the late evolocumab group; 2) between baseline and 4-week follow-up, the percentage of increase in minimum fibrous-cap thickness was significantly greater in the early evolocumab group than in the late evolocumab group; and 3) between baseline and 36-week follow-up, fibrous-cap thickness increased comparably in the early evolocumab group and the late evolocumab group.
Nonculprit lesions in patients with ACS have more vulnerable plaque characteristics compared with those with non-ACS [21]. These vulnerable plaque is easy to occur secondary coronary events in the weeks and months after the onset of ACS [3,4,22].Thus the present study suggested that early therapy with a PCSK9-inhibitor might have a salutary effect for reducing plaque vulnerability in patients with ACS.
Fibrous cap thickness is a major factor in plaque vulnerability [23-25]. The accumulated macrophage abundant in Atheroma can produce proteolytic enzymes (matrix metalloproteinase) capable of degrading the collagen that lends strength to the plaque’s protective fibrous cap, rendering that cap thin, weak, and susceptible to rupture [26,27]. The intense matrix degradation may progressively drive the evolution of an early small Atheroma to a high-risk thincapped Atheroma [21,28]. In this our analysis, increased fibrous cap thickness and decreased macrophage accumulation grade in the early phase (4-week follow-up) after ACS were greater with the early evolocumab treatment than with the late evolocumab treatment in this study. Therefore, the reduction of matrix metalloproteinase release, combined with decreased accumulation of macrophages, might induce fibrous cap thickening with early evolocumab treatment. Previous studies using OCT reported that even statin therapy alone led to a greater increase of fibrous cap thickness in coronary atherosclerotic plaque [8,9,19]. Then, in this study, adding the PCSK9-inhibitor evolocumab to statin therapy on the ultra-early stage showed a more potent effect on increasing fibrous cap thickness in patients with ACS. Recently, the ESCORT (Effect of Early Pitava Statin Therapy on Coronary Fibrous-cap Thickness assessed by Fourier-Domain Optical Coherence Tomography) study in patients with ACS demonstrated that the effect of early statin therapy provided a significant increase in fibrous-cap thickness in coronary plaques [22]. In this trial, pitavastatin 4mg/day (within 24h after the primary PCI) for 3 weeks provided a significant increase in fibrouscap thickness in coronary plaques, while fibrous-cap thickness decreased during the first 3 weeks without pitavastatin. Subsequently, the present our OCT study demonstrated that the percentage of increase in fibrous cap thickness was significantly greater in the earalyevolocumab group between baseline and 4-week follow-up compared with the late evolocumab group. The beneficial effects of early PCSK9-inhibitor on coronary plaques might be expected in a short time as well as early statin therapy. Early initiation of adding the PCSK9-inhibitor evolocumab to statin therapy might further reduce the early secondary coronary events. Our data suggested the effectiveness of early evolocumab therapy in patients with ACS.
In addition, in the ASTEROID trial, very high-intensity statin therapy using rosuvastatin 40mg/dl achieved an average LDL-C of 60.8mg/dL, resulting in significant regression of atherosclerosis for all three pre-specified IVUS measures of disease burden [6]. This trial proposed that treatment of LDL-C levels below the currently accepted guidelines can regress atherosclerosis in patients with coronary disease. Subsequently, a number of studies have reported significant plaque regression induced by intensive lipid-lowering therapy [29,30]. In general, lipid-rich fibroatheromas can grow eccentrically to become very large plaques without obstructing the vessel lumen. These nonobstructive, positively remodeled, advanced plaques are completely silent on traditional stress testing and coronary angiography but portend an imminent risk of plaque rupture [31]. Previous animal experimentation demonstrated that the absence of PCSK9 protects both wild-type and apolipoprotein E-deficient mice from atherosclerosis, whereas it’s over expression is proatherogenic. Furthermore, this study suggested that PCSK9 modulates atherosclerosis mainly via the LDL receptor [32].
Next, regarding lipid arc and lipid length, the lipid rich plaques of longer lipid lengths, wider lipid arcs, and higher degrees of stenos is by OCT were at particularly high risk for future nonculprit lesionrelated cardiac events [33]. We previously reported that the lipid arc was narrower and the lipid length was shorter in the statin plus PCSK9-inhibitor evolocumab group than in the statin alone group at the 12-week follow-up [17]. According to these results, including our present OCT analysis, the PCSK9-inhibitor evolocumab prevented the lipid-rich plaque progression by causing a narrower lipid arc and a shorter lipid length, indicating a reduced extension of atherosclerotic plaque with improved plaque morphology. Moreover, we considered that early therapy with PCSK9-inhibitor is desirable at the earliest possible date. In addition, the sub-analysis of FOURIER trial found that among patients with prior MI, those with a more recent MI, multiple prior MIs, or residual multivessel coronary artery disease were at higher risk of cardiovascular events and tended to experience greater and earlier cardiovascular risk reduction from LDL-C lowering with evolocumab [34]. There also tended to be greater relative risk reductions in cardiovascular outcomes with evolocumab in these high-risk subgroups. Therefore, we expect that very early PCSK9-inhibitor evolocumab therapy in Japanese patients with ACS induces significant plaque regression and, by decreasing plaque lipid content and increasing plaque fibrous cap thickness, induces plaque stabilization.
Study Limitations
There are potential limitations to our data. First, the major limitations of this study are the relatively small number of patients and the short interval duration for follow-up OCT. Second, patients were non-randomly and retrospectively selected in the two centers, so selection bias may have influenced our results. Third, rosuvastatin 5 mg/day is classified as mild-intensity therapy in the USA and Europe. Rosuvastatin 5mg/day is, however, the approved starting dose for aggressive lipid-lowering therapy in Japanese patients [35], who have lower body weights than Caucasians. Fourth, it was reported that OCT was not optimal for detecting Thin-Cap Fibroatheroma (TCFA) since OCT diagnosis was a false positive when TCFA included an accumulation of foam cells, hemosiderin, microcalcifications, and thrombus on the luminal surface. Further studies are needed to elucidate the clinical implications of the changes in fibrous cap thickness measured by OCT. Thus, the combined use of OCT and IVUS might improve TCFA detection accuracy [36,37].
Conclusion
Among patients with ACS who cannot achieve LDL cholesterol targets despite standard lipid lowering therapy, the addition of evolocumab can not only provide a greater reduction of LDL-C even in the short term, but also have a favorable effect on improving incremental growth in fibrous-cap thickness and regression of the lipid-rich plaque. However, a much larger sample size would be useful to confirm this association in a future study.
References
- Baigent C, Keech A, Kearney PM, Blackwell L, Buck G, Pollicino C, et al. Efficacy and safety of cholesterol-lowering treatment: prospective metaanalysis of data from 90,056 participants in 14 randomised trials of statins. Lancet. 2005; 366: 1267-1278.
- Chou R, Dana T, Blazina I, Daeges M, Jeanne TL. Statins for prevention of cardiovascular disease in adults: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2016; 316: 2008-2024.
- Stenestrand U, Wallentin L. Swedish Register of Cardiac Intensive Care (RIKS-HIA). Early statin treatment following acute myocardial infarction and 1-year survival. JAMA. 2001; 285: 430-436.
- Schwartz GG, Olsson AG, Ezekowitz MD, Ganz P, Oliver MF, Waters D, et al. Effects of atorvastatin on early recurrent ischemic events in acute coronary syndromes: the MIRACL study: A randomized controlled trial. JAMA. 2001; 285: 1711-1718.
- Nissen SE, Tuzcu EM, Schoenhagen P, Brown BG, Ganz P, Vogel RA, et al. Effect of intensive compared with moderate lipid-lowering therapy onprogression of coronary atherosclerosis: A randomized controlled trial. JAMA. 2004; 291: 1071-1080.
- Nissen SE, Nicholls SJ, Sipahi I, Libby P, Raichlen JS, Ballantyne CM, et al. Effect of very high-intensity statin therapy on regression of coronary atherosclerosis: The ASTEROID trial. JAMA. 2006; 295: 1556-1565.
- Kim Y, Johnson TW, Akasaka T, Jeong MH. The role of optical coherence tomography in the setting of acute myocardial infarction. J Cardiol. 2018; 72: 186-192.
- Hattori K, Ozaki Y, Ismail TF, Okumura M, Naruse H, Kan S, et al. Impact of statin therapy on plaque characteristics as assessed by serial OCT, grayscale and integrated backscatter-IVUS. JACC Cardiovasc Imaging. 2012; 5: 169- 177.
- Takarada S, Imanishi T, Kubo T, Tanimoto T, Kitabata H, Nakamura N, et al. Effect of statin therapy on coronary fibrous-cap thickness in patients with acute coronary syndrome: assessment by optical coherence tomography study. Atherosclerosis. 2009; 202: 491-497.
- Teramoto T, Uno K, Miyoshi I, Khan I, Gorcyca K, Sanchez RJ, et al. Lowdensity lipoprotein cholesterol levels and lipid-modifying therapy prescription patterns in the real world: an analysis of more than 33,000 high cardiovascular risk patients in Japan. Atherosclerosis. 2016; 251: 248-254.
- Koren MJ, Lundqvist P, Bolognese M, Neutel JM, Monsalvo ML, Yang J, et al. Anti-PCSK9 monotherapy for hypercholesterolemia: the MENDEL-2 randomized, controlled phase III clinical trial of evolocumab. J Am Coll Cardiol. 2014; 63: 2531-2340.
- Urban D, Pöss J, Böhm M, Laufs U. Targeting the Proprotein convertasesubtilisin/kexin type 9 for the treatment of dyslipidemia and atherosclerosis.J Am Coll Cardiol. 2013; 62: 1401-1408.
- Wu CY, Tang ZH, Jiang L, Li XF, Jiang ZS, Liu LS. PCSK9 siRNA inhibitsHUVEC apoptosis induced by ox-LDL via Bcl/Bax-caspase9-caspase 3 pathway. Mol Cell Biochem. 2012; 359: 347-358.
- Nicholls SJ, Puri R, Anderson T, Ballantyne CM, Cho L, Kastelein JJ, et al. Effect of evolocumab on progression of coronary disease in statin-treated patients: The GLAGOV Randomized Clinical Trial. JAMA. 2016; 316: 2373- 2384.
- Sabatine MS, Giugliano RP, Keech AC, Honarpour N, Wiviott SD, Murphy SA, et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017; 376: 1713-1722.
- Schwartz GG, Steg PG, Szarek M, Bhatt DL, Bittner V, Diaz R, et al. Alirocumab and Cardiovascular Outcomes after Acute Coronary Syndrome. N Engl J Med. 2018; 379: 2097-2107.
- Yano H, Horinaka S, Ishimitsu T. Effect of evolocumab therapy on coronary fibrous cap thickness assessed by optical coherence tomography in patients with acute coronary syndrome. J Cardiol. 2020; 75: 289-295.
- Tearney GJ, Regar E, Akasaka T, Adriaenssens T, Barlis P, Bezerra HG, et al. Consensus standards for acquisition, measurement, and reporting of intravascular optical coherence tomography studies: A report from the International Working Group for Intravascular Optical Coherence Tomography Standardization and Validation. J Am Coll Cardiol. 2012; 59: 1058-1072.
- Komukai K, Kubo T, Kitabata H, Matsuo Y, Ozaki Y, Takarada S, et al. Effect of atorvastatin therapy on fibrous-cap thickness in coronary atherosclerotic plaque as assessed by optical coherence tomography: the EASY-FIT study. J Am Coll Cardiol. 2014; 64: 2207-2217.
- Tahara S, Morooka T, Wang Z, Bezerra HG, Rollins AM, Simon DI, et al. Intravascular optical coherence tomography detection of atherosclerosis and inflammation in murine aorta. Arterioscler ThrombVasc Biol. 2012; 32: 1150- 1157.
- Kato K, Yonetsu T, Kim SJ, Xing L, Lee H, McNulty I, et al. Nonculprit plaques in patients with acute coronary syndromes have more vulnerable features compared with those with non-acute coronary syndromes: A 3-vessel optical coherence tomography study. Circ Cardiovasc Imaging. 2012; 5: 433-440.
- Nishiguchi T, Kubo T, Tanimoto T, Ino Y, Matsuo Y, Yamano T, et al. Effect of Early Pitavastatin Therapy on Coronary Fibrous-Cap Thickness Assessed by Optical Coherence Tomography in Patients With Acute Coronary Syndrome: The ESCORT Study. JACC Cardiovasc Imaging. 2018; 11: 829-838.
- Virmani R, Kolodgie FD, Burke AP, Farb A, Schwartz SM. Lessons from sudden coronary death: A comprehensive morphological classification scheme for atherosclerotic lesions. Arterioscler Thromb Vasc Biol. 2000; 20: 1262-1275.
- Tian J, Ren X, Vergallo R, Xing L, Yu H, Jia H, et al. Distinct morphological features of ruptured culprit plaque for acute coronary events compared to those with silent rupture and thin-cap fibroatheroma: A combined optical coherence tomography and intravascular ultrasound study. J Am Coll Cardiol. 2014; 63: 2209-2216.
- Burke AP, Farb A, Malcom GT, Liang YH, Smialek J, Virmani R. Coronary risk factors and plaque morphology in men with coronary disease who died suddenly. N Engl J Med. 1997; 336: 1276-1282.
- Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation. 2002; 105: 1135-1143.
- Moreno PR, Purushothaman KR, Fuster V, Echeverri D, Truszczynska H, Sharma SK, et al. Plaque neovascularization is increased in ruptured atherosclerotic lesions of human aorta: Implications for plaque vulnerability. Circulation. 2004; 110: 2032-2038.
- Chatzizisis YS, Baker AB, Sukhova GK, Koskinas KC, Papafaklis MI, Beigel R, et al. Augmented expression and activity of extracellular matrix-degrading enzymes in regions of low endothelial shear stress colocalize with coronary atheromata with thin fibrous-caps in pigs. Circulation. 2011; 123: 621-630.
- Nicholls SJ, Ballantyne CM, Barter PJ, Chapman MJ, Erbel RM, Libby P, et al. Effect of two intensive statin regimens on progression of coronary disease. N Engl J Med. 2011; 365: 2078-2087.
- Nicholls SJ, Hsu A, Wolski K, Hu B, Bayturan O, Lavoie A, et al. Intravascular ultrasound-derived measures of coronary atherosclerotic plaque burden and clinical outcome. J Am Coll Cardiol. 2010; 55: 2399-2407.
- Moreno PR. The high-risk thin-cap fibroatheroma: A new kid on the block. Circ Cardiovasc Interv. 2009; 2: 500-502.
- Denis M, Marcinkiewicz J, Zaid A, Gauthier D, Poirier S, Lazure C, et al. Gene inactivation of Proprotein convertase subtilisin/kexin type 9 reduces atherosclerosis in mice. Circulation. 2012; 125: 894-901.
- Xing L, Higuma T, Wang Z, Aguirre AD, Mizuno K, Takano M, et al. Clinical significance of lipid-rich plaque detected by optical coherence tomography: A 4-year follow-up study. J Am Coll Cardiol. 2017; 69: 2502-2513.
- Sabatine MS, De Ferrari GM, Giugliano RP, Huber K, Lewis BS, Ferreira J, et al. Clinical benefit of evolocumab by severity and extent of coronary artery disease. Circulation. 2018; 138: 756-766.
- Ogawa H, Matsui K, Saito Y, Sugiyama S, Jinnouchi H, Sugawara M, et al. Differences between rosuvastatin and atorvastatin in lipid-lowering action and effect on glucose metabolism in Japanese hypercholesterolemic patients with concurrent diabetes–lipid-lowering with highly potent statins in hyperlipidemia with type 2 diabetes patients (LISTEN) Study. Circ J. 2014; 78: 2512-2515.
- Fujii K, Hao H, Shibuya M, Imanaka T, Fukunaga M, Miki K, et al. Accuracy of OCT, grayscale IVUS, and their combination for the diagnosis of coronary TCFA: an ex vivo validation study. JACC Cardiovasc Imaging. 2015; 8: 451- 460.
- Fujii K, Kawakami R, Hirota S. Histopathological validation of optical coherence tomography findings of the coronary arteries. J Cardiol. 2018; 72: 179-185.