
Case Report
Austin J Clin Cardiolog. 2022; 8(2): 1095.
Enormous Pulmonary Arteries and a Late Diagnosis of a Common Congenital Cardiac Issue: A Case Report
Murphy G* and Shiels P
Department of Cardiology, Tullamore Regional Hospital, Tullamore, Co Offaly, Ireland
*Corresponding author: Murphy G, Department of Cardiology, Tullamore Regional Hospital, Co Offaly, Ireland
Received: August 04, 2022; Accepted: August 30, 2022; Published: September 06, 2022
Abstract
Background: Noonan’s syndrome is the second most common congenital cardiac syndrome after Down syndrome. Classically it is associated with pulmonary stenosis, Hypertrophic Cardiomyopathy (HCM), and Atrial Septal Defects (ASD). Late presentations, milder phenotypes and atypical presentations can occur in adult life and have cardiovascular implications.
Case Summary: A 62-year-old presented to the emergency department with chest pain and atrial fibrillation with fast ventricular rate and RBBB morphology. A transthoracic echocardiogram demonstrated a massive Pulmonary Artery (PA), concerning for pulmonary hypertension and an atrial septal defect (ASD). Right heart catheterization indicated enormous coronary artery ectasia but normal PA pressures and wedge pressures. He was identified as a late presentation of Noonan’s syndrome on subsequent genetic testing.
Discussion: Some series indicate a prevalence of 1 in 100 for mild Noonan’s syndrome phenotypes, highlighting the importance of understanding its cardiac presentations. In this case, we present a man with minimal symptoms but gross pathologies of his pulmonary and coronary arteries. Furthermore, pulmonary artery dilatation without pulmonary valve pathology has not been reported in the literature, and only a few reports of coronary artery ectasia are reported.
Learning Points:
• Noonan’s syndrome should be considered in adult life
• An atypical presentation of Noonan’s syndrome on imaging
• Imaging review
• Coronary artery ectasia
Keywords: Noonan’s syndrome; Pulmonary artery; Coronary artery ectasia; ASD
Introduction
Noonan’s syndrome, first reported by Noonan et al. in 1963, is an inherited chromosomal condition affecting 1 in every 2500 births [1]. Characteristics include a webbed neck, failure to thrive, short stature, low set ears, testicular and renal abnormalities. Cardiac involvement is frequent (80%) and predominantly presents with pulmonary stenosis (39%), hypertrophic cardiomyopathy (10%), ASD (8%), Tetralogy of Fallot (4%), and left-sided lesions, [2]. It is the second most common syndrome causing cardiac abnormalities [3].
Diagnosis is challenging; milder phenotypes may be as common as one per 100 live births and present late [1,4]. Furthermore, phenotypic differences limit the accuracy of genetic testing to 70% [3]. It thus remains useful for Cardiologists to identify clinical features of milder phenotypes on presentation to their clinic asguideline-based management for people with Noonan’s syndrome should be initiated early [5].
Timeline
Presented to hospital October with chest pain and atrial fibrillation 2016.
TOE Identifies enlarged PA and ASD.
Referral for right heart catheterization and proceeded to angiography January 2018.
Decision made not to close ASD, January 2018.
Reviewed in clinic August 2019, improvement in dyspnoea.
Case Presentation
A 62-year-old presented to the hospital in October 2016 with a two-day history of left-sided chest pain, dyspnoea and palpitations. His background included COPD, TB, and rheumatoid arthritis.
12 lead ECG showed atrial fibrillation with RBBB and rt axis deviation without ischemia.
The patient was cyanotic and in atrial fibrillation with a rapid ventricular rate. Examination demonstratedbi-basal crackles, no pedal oedema and no visible JVP. Troponin was normal. Chest X-ray showed blunting of the costophrenic angles bilaterally. Transthoracic echo showed an enlarged left atrium with good ventricular function, a dilated Pulmonary Artery (PA), and a colour jet across the intra atrial septum. He was started on bisoprolol 10mg and apixaban 5 mg. He was discharged with a Transoesophageal Echo (TOE) as an outpatient. TOE elicited a small secundum atrial septal defect of 0.5cm with disproportional large pulmonary artery dilatation and normal biventricular function Video 1. (TOE)
Cardiac MRI identified an enormous pulmonary artery suggesting pulmonary hypertension out of proportion to the ASD shunt (Figure 1).

Figure 1:
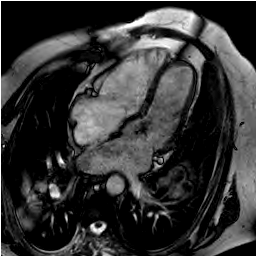
Figure 2:
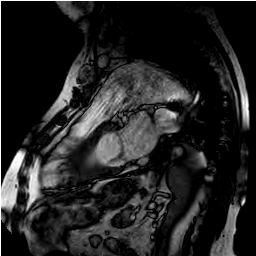
Figure 3:
The patient was referred for further interventional opinion and consideration for percutaneous closure. Clinically he remained well on a rate and anticoagulation approach He proceeded to right heart catheterization, on which he had preserved cardiac output. Pulmonary artery pressure remained within the normal range at 25mmhg with a wedge pressure of 20mmhg despite the enlarged pulmonary artery.
Coronary angiography was performed and showed enormous atypical ectatic coronary.
He was further initiated on calcium channel blockers and continued diuretics and beta blockers. Subsequently, he was sent for genetic testing and returned a positive genetic profile for Noonan’s syndrome. He was managed medically, and his exercise tolerance and dyspnoea improved from NYHA III on presentation to NYHA II with no intervention needed.
Discussion
Noonan’s syndrome is the second most common congenital presentation of cardiac anomalies after Down syndrome, and its incidence could be as high as 1 in every 100 live births [1].Variable penetrance and late cardiac presentations of Noonan’s syndrome have been reported [1,4]. Our case demonstrates a man presenting with no symptoms for the previous six decades despite gross abnormalities on imaging.
While Pulmonary artery stenosis and HCM are the most common abnormalities associated with Noonan’s syndrome, this case demonstrates normal valvular function with an isolated enormous pulmonary artery. Furthermore, the large ectatic coronary arteries in Noonan’s syndrome are very rare and infrequently reported [6,7]. Best practice management is unknown.
Survival outcome data on this patient cohort is scarce. Studies conclude a diagnosis of HCM in Noonan’s syndrome is associated with worse outcomes [8,9]. One study following patients for 14 years demonstrated 1% of patients to have died from heart failure, all with a diagnosis of HCM, indicating other cardiac abnormalities in Noonan’s syndrome to be less fatal [9].
Treatment of these patients often involves interventional approaches; one-third progress to either open valve surgery or percutaneous valvuloplasty, predominantly on a stenotic pulmonary valve [10]. This highlights a need for early diagnosis and monitoring of these patients for timely treatment.
Conclusion
We present a case of a 62-year-old presenting for the first time in adult life with marked abnormalities on imaging. The pulmonary artery dilatation without pulmonary stenosis and diffuse coronary ectasia are rare, infrequently reported presentations of a syndrome which commonly presents to cardiologists.
References
- Mendez HM, Opitz JM. Noonan syndrome: a review. American journal of medical genetics. 1985; 21: 493-506.
- Marino B, Digilio MC, Toscano A, Giannotti A, Dallapiccola B. Congenital heart diseases in children with Noonan syndrome: An expanded cardiac spectrum with high prevalence of atrioventricular canal. The Journal of pediatrics. 1999; 135: 703-706.
- Allanson JE. Noonan syndrome. J Med Genet. 1987; 24: 9-13.
- Fiore M, Castet S-M, Bordet J-C, Naudion S, Alessi M-C. Atypical late diagnosis of Noonan syndrome revealed by bleedings due to platelet dysfunction. Journal of Thrombosis and Thrombolysis. 2021.
- Romano AA, Allanson JE, Dahlgren J, Gelb BD, Hall B, Pierpont ME, et al. Noonan Syndrome: Clinical Features, Diagnosis, and Management Guidelines. Pediatrics. 2010; 126: 746-759.
- Uçar T, Atalay S, Tekin M, Tutar E. Bilateral Coronary Artery Dilatation and Supravalvular Pulmonary Stenosis in a Child with Noonan Syndrome. Pediatric Cardiology. 2005; 26: 848-850.
- Ly R, Soulat G, Iserin L, Ladouceur M. Coronary artery disease in adults with Noonan syndrome: Case series and literature review. Archives of cardiovascular diseases. 2021; 114: 598-605.
- Calcagni G, Limongelli G, D’Ambrosio A, Gesualdo F, Digilio MC, Baban A, et al. Cardiac defects, morbidity and mortality in patients affected by RASopathies. CARNET study results. International journal of cardiology. 2017; 245: 92-98.
- Colquitt JL, Noonan JA. Cardiac findings in Noonan syndrome on long-term follow-up. Congenital heart disease. 2014; 9: 144-150.
- Shaw AC, Kalidas K, Crosby AH, Jeffery S, Patton MA. The natural history of Noonan syndrome: a long-term follow-up study. Archives of Disease in Childhood. 2007; 92: 128-132.