
Research Article
Austin J Clin Cardiolog. 2023; 9(1): 1103.
Effects of High-Fat Feeding on Expression of Genes Regulating Fatty-1 Acid Metabolism in Hearts of Pregnant C57BL/6 Mice
Dudick K, Chen C, Shoemaker R*
Department of Dietetics and Human Nutrition, University of Kentucky, USA.
*Corresponding author: Robin Shoemaker Assistant Professor, Department of Dietetics and Human Nutrition, University of Kentucky, Kentucky.
Received: December 06, 2022; Accepted: February 13, 2023; Published: February 20, 2023
Abstract
Cardiovascular complications in pregnancy increase risk for subsequent heart disease, suggesting adverse events during pregnancy may permanently alter maternal heart health. The heart undergoes physiologic hypertrophy with pregnancy, which is distinct from pathological remodeling associated with obesity. We previously demonstrated that C57BL/6 mice fed a high-fat diet, a model of diet-induced obesity, had attenuated cardiac hypertrophy with pregnancy compared to low-fat controls, associated concentric remodeling. Dual effects of pregnancy and obesity on cardiac metabolism during hypertrophy have not been studied. We investigated whether expression of genes regulating fatty acid metabolism in the heart were altered in pregnant mice fed a high-fat diet. The Nanostring Metabolic Pathways Panel and nCounter analysis system was used to quantify individual mRNA transcripts of genes regulating fatty acid metabolism from the left ventricles of pregnant and non-pregnant female C57BL/6 mice fed a high-fat or control low-fat diet. Pregnancy increased expression of genes regulating fatty acid transport (Cd36, Slc27a1, Cpt1b) and β-oxidation (Acaa2, Acadl, Acox1, Ehhadh, Mlycd), but the effect was observed in low-fat mice only. Increases in gene expression with high-fat feeding were pronounced in non-pregnant mice, but effects not additive with pregnancy. Further, three genes with functions related to energy metabolism (Glul, Mat2a, Ogdhl,) were increased in low fat–fed pregnant mice only. Obesity during pregnancy may “max out” cardiac fatty acid utilization through upregulation of transporters and oxidation of long-chain fatty acids, and also downregulate metabolic pathways essential to cardiac adaptation. These 48 results suggest pre-existing obesity could disrupt cardiac physiologic remodeling during pregnancy.
Keywords: Maternal; Cardiac hypertrophy; Obesity; Metabolism; Cardiac; Cardiovascular disease; Pregnancy
Introduction
Pregnancy requires profound adaptation of the cardiovascular system. Left Ventricular (LV) mass is increased by 25% during pregnancy to accommodate the increased blood volume necessary for the growing fetoplacental unit and to meet the increased metabolic demands of the mother [1]. Cardiac remodeling occurring during pregnancy (physiological remodeling) is assumed to be transient, and is reversed in healthy pregnancy [2]. Physiological remodeling is distinct from remodeling that occurs due to pathologic stressors, such as elevated blood pressure and obesity [3,4]. Cardiovascular complications are the leading cause of maternal death in the United States [5], suggesting that pathologic stressors during pregnancy may adversely affect normal physiologic remodeling.
Obesity is an independent risk factor for cardiovascular disease [6]. Obesity in humans is associated with increased LV mass [7] and concentric remodeling [8]; both of these factors are associated with poor cardiovascular prognosis. Obesity also strongly increases risk for pregnancy complications [9], and several studies in humans demonstrate that cardiac function during pregnancy is impaired in women with obesity [10,11]. We previously demonstrated that HF-feeding of mice during pregnancy promoted concentric remodeling of the left ventricle, compared to LF-fed mice where pregnancy was associated with eccentric remodeling [12]. Moreover, after 10 weeks postpartum, mice fed a HF-diet during pregnancy had augmented cardiac hypertrophy compared to LF-fed controls, suggesting that alterations of cardiac geometry during pregnancy mediated by HF-feeding had a lasting effect [13].
Mechanisms linking obesity during pregnancy with adverse cardiac remodeling are not well understood. A recent study in Ldlr-/- mice fed a Western diet (a mouse model of metabolic syndrome) demonstrated that pregnant mice exhibited cardiac hypertrophy and fibrosis, compared to pregnant mice fed a control diet, associated with crosstalk between cardiac fibrosis and lipid metabolism pathways [14]. Aberrant cardiac metabolism is a hallmark of disease in patients with heart failure and diabetes mellitus. Normally quite flexible, the heart can adapt to an altered metabolic state by selecting available substrates for the most effective generation of ATP. This flexibility is lost with impaired heart function resulting from diabetes or obesity; fatty acid utilization is increased and glucose oxidation rates are decreased compared to a healthy heart [15]. Since cardiac metabolism is also altered during pregnancy, similarly shifting to rely primarily on utilization of fatty acids [16], we hypothesized that HF-feeding during pregnancy may adversely impact changes in cardiac metabolism normally occurring in pregnancy. In the current study, we investigated the effect of HF-feeding during pregnancy on expression of genes regulating cardiac fatty acid metabolism.
Methods
Experimental animals
Animal studies were approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Kentucky and were conducted in accordance with the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals. Female C57BL/6J mice (8 weeks of age; Jackson Laboratory, Bar Harbor, ME, stock # 000664) were randomly assigned to receive, ad libitum, either a high fat (HF; 60% kcal from fat; D12492, Research Diets, New Brunswick, NJ) or a control low fat (LF, 10% kcal from fat; D12450B, Research Diets Inc) diet for 8 weeks. The control LF diet was purified and ingredient-matched to the HF diet, and the fat source for both diets was soybean oil and lard (where lard comprises the excess fat in the HF diet). The energy densities of the LF and HF diet are 3.82 and 5.21 kcal/g, respectively.
At 8 weeks of diet feeding, all female mice were placed in a cage with male mice of the same strain and diet. After 2 days, females were removed from the males, and placed in single housing for the duration of the study. On gestational day 18, heart function was assessed via echocardiography and results were published separately [12]. The following day, mice were anesthetized with ketamine/xylazine (100/10 112 mg/kg, i.p.) for exsanguination, saline perfusion, and excision of hearts. Hearts were snap frozen in liquid nitrogen and stored at -80oC until analysis.
Extraction of RNA and gene expression analysis of cardiac tissue
Total RNA was extracted from approximately 20 mg of left ventricle of n= 8 LF non-pregnant, n=9 LF pregnant, n=9 HF non-pregnant, and n=10 HF pregnant mice using the Maxwell RSC (Promega, Madison, WI). The concentration of RNA and the purity was assessed using a Nanodrop 2000. All samples had a 260/280 and 260/230 ratio that was greater than 2.0. The abundance of mRNA of genes regulating metabolism was determined via the Nanostring nCounter Metabolic Pathways Panel and nCounter Analysis System (NanoString Technologies, Seattle, WA). The nCounter Analysis system was available at the University of Kentucky Genomics Core Laboratory. Described previously [13], the Nanostring nCounter gene expression system is a multiplexed assay that uses a combination of unique capture probes and color-coded reporter probes to capture and count individual mRNA transcripts with high sensitivity and tight correlation to qRT-PCR [17]. Fifty nanograms of RNA of each sample was hybridized to the target-specific capture and reporter probes in the CodeSet according to the manufacturer’s instructions. Samples were cooled to 4oC, loaded into nCounter SPRINT cartridges, and then analyzed using the nCounter Gene Expression Assay. Raw data were normalized by creating scaling factors for the sum of the positive controls and the geometric mean of the four housekeeping genes. Data represent the mean of normalized counts.
Statistical analysis
Data are presented as mean ± SEM. Statistical analyses were performed using SigmaPlot version 12.3. All data passed normality or equal variance tests or logarithmic transformation was used to achieve normality. Two-tailed Student’s t-tests were used for analysis of data between two groups. For two-factor analysis, a two-way ANOVA was used to analyze end-point measurements with between-group factors of pregnancy and diet, followed by Holm-Sidak for post hoc pairwise analyses. Values of P<0.05 were considered to be statistically significant.
Results
Heart weights of LF-and HF-fed pregnant and non-pregnant mice
We previously reported that body weight was increased by both pregnancy and HF-feeding, with body weight of HF-fed pregnant mice approximately 5 grams (12%) greater than LF-fed pregnant mice [12]. Here, we report an overall effect of HF-feeding to increase wet heart weight in both pregnant and non-pregnant mice (P<0.05), but no effect of pregnancy (Figure 1A). When considering the ratio of wet heart weight to body weight (Figure 1B), there was a similar effect of HF-feeding in non-pregnant mice (P<0.0001), and also an effect of pregnancy in both diet groups (P<0.0001).
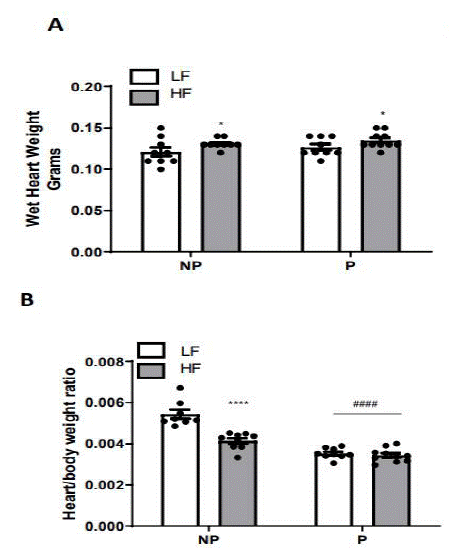
Figure 1: Heart weight of pregnant and non-pregnant mice fed a LF- or HF-diet. (A) Wet heart weight. (B) Heart/body weight. Data are mean + SEM from n=8 LF non-pregnant, n=9 LP Pregnant, n=18 HF non-pregnant, and n=12 HF pregnant. **, P<0.01 effect of diet; ***, P<0.001 effect of pregnancy analyzed by two-way ANOVA followed by Holm-Sidak pairwise analysis.
Expression of cardiac genes regulating fatty acid metabolism
To determine the effects of pregnancy and HF-feeding on expression of genes involved in cardiac metabolism, mRNA abundance of genes in the Nanostring nCounter Metabolic Pathways Panel was quantified from left ventricles of LF- and HF-fed pregnant and non-pregnant mice. Using two-way ANOVA with pairwise comparisons, we identified forty seven genes with a significant effect (P<0.01) of either diet or pregnancy. Of these, nine were involved in fatty acid regulation. Genes involved in fatty acid transport were: fatty acid binding protein 3 (Fabp3), fatty acid transport protein (Cd36), carnitine palmitoyltransferase 1b (Cpt1b), and solute carrier family 27 member 1 (Slc27a1). Genes involved in β-oxidation were acetyl-CoA acyltransferase 2 (Acaa2), acyl-CoA oxidase 1 (Acox1), long-chain acyl-CoA dehydrogenase (Acadl), enoyl-CoA, hydratase/3-hydroxyacyl CoA dehydrogenase (Ehhadh), and malonyl CoA decarboxylase (Mlycd).
Figure 2A-H demonstrates that all of these genes, with the exception of Fabp3, were increased with pregnancy, but the effect was only significant in LF-fed mice. The genes Acaa2, Acadl, and Cptb1 were significantly increased with HF-feeding in both pregnant and non-pregnant mice (Figure 2A, 2B, 161 2C). In contrast, the genes Acox1, Cd36, Slc27a1, and Mlcyd were increased with HF-feeding only in non-pregnant mice (Figure 2D, 2E, 2F, 2G), and pairwise analysis revealed no effect of HF-feeding in pregnant mice on gene expression of Acox1 (p=0.108), Cd36 (p=0.328), Slc27a1 (p=0.999), or Mlycd (p=0.971). The expression of the gene Ehhadh increased with pregnancy in LF-fed mice, but not HF-fed mice (p=0.180), and further, was significantly decreased by HF-feeding in pregnant mice (Figure 2H, 166 p<0.045). For Fabp3, there was an overall effect of HF diet to increase the abundance of cardiac Mrna (P<0.01), but no effect of pregnancy (LF, NP: 36,189 + 1420; LF, P: 40,191 + 1983, HF NP: 50,511 + 168 4087, HF P: 51,460 + 3696 normalized mRNA counts).
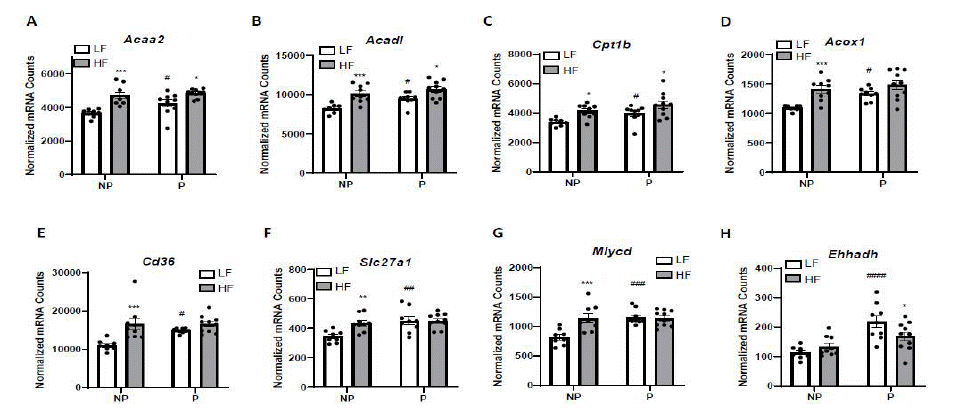
Figure 2: mRNA abundance of fatty acid utilization genes in pregnant and non-pregnant mice fed a LF or HF diet. Genes were measured using Nanostring nCounter from 20 mg of left ventricles: A) Acaa2, B) Acadl, C) Cpt1b, D) Acox1, E) Cd36, F) Slc27a1, G) Mlycd H) Ehhadh. Data are expressed as counts of mRNA transcripts, normalized to the geometric mean of counts of four housekeeping genes. Data are mean + SEM from n= 8 (LF NP), n=9 (LF P), n=9 (HF NP), and n=10 (HF, P). *, P<0.05 effect of diet; **, P<0.01 effect of diet; ***, P<0.001 effect of diet; #, P<0.05 effect of pregnancy, ##, P<0.01 effect of pregnancy; ###, P<0.001 by two-way ANOVA followed by Holm-Sidak pairwise analysis.
In addition to genes regulating fatty acid utilization, we identified four genes for which there was a significant interaction (P<0.01) among diet and pregnancy, and where expression in pregnancy was significantly reduced in HF-fed compared to LF-fed pregnant mice (Figure 3A-D): glutamate-ammonia ligase (Glul), methionine adenosyltransferase 2a (Mat2a), and oxoglutarage dehydrogenase L (Ogdhl).
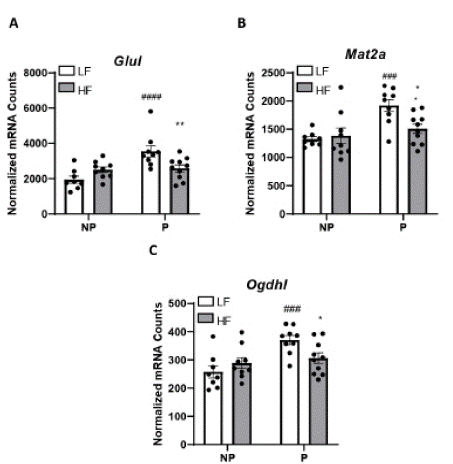
Figure 3: Expression of cardiac genes increased in LF-fed but not HF-fed mice. Genes were measured using Nanostring nCounter from 20 mg of left ventricles: A) Glul, B) Mat2a, C) Ogdhl. Data are expressed as counts of mRNA transcripts, normalized to the geometric mean of counts of four housekeeping genes. Data are mean + SEM from n= 8 (LF NP), n=9 (LF P), n=9 (HF NP), and n=10 (HF, P). *, P<0.05 effect of diet; **, P<0.01 effect of diet; ###, P<0.001 effect of pregnancy; ####, P<0.0001 by two-way ANOVA followed by Holm-Sidak pairwise analysis.
Discussion
Obesity significantly increases risk for cardiovascular disease. Over half of reproductive-age women in the United States have obesity [18]. Since cardiovascular health during pregnancy may have a lifetime impact on maternal cardiovascular risk [19], knowledge about the impacts of obesity on heart function during pregnancy is urgently needed. The present study examined the effects of HF-feeding on expression of genes regulating fatty acid metabolism in the heart during pregnancy. The major findings from this study are 1) Expression of cardiac genes regulating fatty acid uptake and transport (Fabp3, Cpt1b, Cd36, Slc27a1, and Mlycd) were increased with HF-feeding and (except for Fabp3) with pregnancy; however effects were not additive, where generally, HF-fed pregnant did not exhibit marked increase in gene expression compared to LF-fed pregnant mice. 2) Some genes involved in β-oxidation of long-chain fatty acids (Acaa2 and Acadl) were increased by both pregnancy and HF-feeding, but Ehhadh (involved in the production of medium-chain dicarboxylic acids) was increased only in LF-fed pregnant mice. 3) HF-fed pregnant mice had reduced expression of three non-fatty acid –related genes with roles in metabolism: Glul, Mat2a, and Ogdhl. Results indicate that both pregnancy and HF-feeding increased expression of genes regulating fatty acid utilization in left ventricles, but the effects were not additive in HF-fed pregnant mice.
Obesity and dyslipidemia are risk factors for cardiac hyperFigure trophy, a predictor of adverse outcomes [20]. Pregnancy, a condition of rapid weight gain and elevated serum lipid status, also induces cardiac hypertrophy, but this is not associated with cardiovascular disease. We previously demonstrated that mice fed a LF diet during pregnancy exhibited cardiac hypertrophy [12]. Similar to existing literature in humans [21] and rodents [2], this was associated with a change in cardiac geometry, where the ventricle chamber was larger and the cardiac wall thinner compared to LF non-pregnant mice. This reduction in Relative Wall Thickness (RWT) is termed “eccentric” remodeling. In contrast, HF-fed pregnant mice did not exhibit an increase in LV mass compared to HF-fed non-pregnant mice. Further, HF-fed pregnant mice exhibited increased RWT (termed “concentric” remodeling) compared to LF-fed pregnant mice. Increased RWT and concentric remodeling, is an indicator of impaired cardiac function. These data suggest that HF-feeding during pregnancy promotes adverse cardiac remodeling. In the current study, we demonstrate increased expression of genes regulating fatty acid utilization in left ventricles of LF-fed pregnant compared to non-pregnant mice, suggesting that increased energy metabolism pathways could promote physiological cardiac hypertrophy of pregnancy. Fatty acid utilization genes were generally increased with HF-feeding in non-pregnant mice, but not further augmented with pregnancy. Given previous findings of impaired cardiac hypertrophy and adverse remodeling in HF-fed pregnant mice [12], we propose that HF-feeding during pregnancy may impair cardiac energy metabolism pathways involved in promoting physiological cardiac hypertrophy of pregnancy.
Consistent with studies in ob/ob mice [22], a mouse model of genetic obesity, and a recent paper of HF-feeding in C57BL/6 mice [23] we report that HF-feeding increased expression of genes regulating fatty acid transport into the mitochondria (Cd36, Fabp3, Slc27a1, Cpt1b, Mlycd) and fatty acid β-oxidation (Acaa2, Acadl, Acox1). With the exception of Fabp3, these genes were increased with pregnancy in LF-fed mice. These data indicate that both pregnancy and obesity promote increased fatty acid utilization as a substrate in the heart, with obesity having the larger influence. However, in general these effects were not additive in the HF-fed pregnant mice; the mRNA count of these genes in HF-fed pregnant mice were roughly equivalent to those in the HF-fed non-pregnant mice. This suggests that obesity may “max out” fatty acid utilization through upregulation of transporters and oxidation of long- and very long-chain fatty acids.
Ehhadh encodes a protein that is part of the classical peroxisomal fatty acid β-oxidation pathway, with an essential role in the production of medium-chain dicarboxylic acids [24]. The purpose of medium chain dicarboxylic acid production in cardiac metabolism is not known, but changes in the expression of this gene have been reported in hearts of patients with mitral regurgitation and aortic valve disease [25]. In the current study, the expression level in the HF- pregnant mice was significantly reduced compared to that of the LF-pregnant mice, suggesting a role for this gene in cardiac metabolism of pregnancy that was impaired with obesity. Dicarboxylic acids can be broken down to yield acetyl-CoA and succinyl-CoA as products, important intermediates for the tricarboxylic acid cycle [26]. Exogenous medium chain fatty acids serve as a rapid energy source because they are metabolized quickly, and might be an important substrate for the heart during pregnancy, when the heart’s energy needs are dramatically increased. Supplementation of medium-chain fatty acids improved cardiac function in rats under conditions where oxidation of fatty acids was impaired [27]. Further, medium-chain fatty acid supplementation in the diets of rats with left ventricular hypertrophy reduced hypertrophy and cardiac oxidative stress [28,29]. Findings from our current study extend those of published literature by suggesting increased Ehhadh cardiac expression in pregnancy may be a mechanism contributing the increased metabolic flexibility of the heart during pregnancy, where utilization of medium chain dicarboxylic acids as fuel during pregnancy may be impaired by HF-feeding.
Ogdhl encodes an enzyme of the 2-oxoglutarate dehydrogenase multienzyme complex, which decarboxylates alpha-ketoglutarate in the tricarboxylic acid cycle. A recent study investigating genes involved in fetal heart maturation in mice identified changes in cardiac fatty acid metabolism to be characteristic of cardiomyocyte progression to the adult phenotype [30]. Changes in Ogdhl expression was found to be the most dramatic change between neonatal and adult heart, indicating Ogdhl to be a critical regulator of energy metabolism maturation in cardiomyocytes. Published literature demonstrates upregulation of genes predominately expressed during fetal cardiac development occurs during cardiac hypertrophy [31]. In the current study, Ogdhl expression was dramatically increased in hearts of pregnant LF-fed, but not HF-fed mice, suggesting Ogdhl is a critical mediator of physiologic hypertrophy of pregnancy.
In the current study, expression of Glul was increased in LF-fed, but not HF-fed hearts during pregnancy. Glul catalyzes the synthesis of glutamine from glutamate and ammonia, important for cellular detoxification, and playing an important role in biosynthesis of amino acids. Genetic studies revealed that the polymorphism rs10911021, located upstream of the noncoding sequence of Glul, to be strongly linked to morbidity and mortality of cardiovascular disease in patients with type 2 diabetes. In a study including 1,517 cases of coronary heart disease and 2,671 non-coronary heart disease controls, rs10911021 risk allele homozygotes exhibited a 32% decrease in the expression of the Glul gene in human endothelial cells [32]. In a recent study examined genes involved in metabolism in hearts from humans with or without acute myocardial infarction, and Glul was one of three genes identified to be significantly increased in with myocardial infarction [33], which might be increased in a compensatory manner. Findings from the current study extend previous literature by demonstrating that Glul expression in the heart contributes to physiological hypertrophy in pregnancy.
Mat2a encodes the gene S-adenosylmethionine (a key methyl donor in cellular processes) from methionine and ATP. Results from our study indicate gene expression of Mat2a increases during pregnancy, but not in mice fed a HF-diet. Accumulation of cellular ammonia can cause cardiac injury, and a recent study demonstrated administration of ammonia to pigs resulted in changes in genes regulating cardiac metabolism, such as Cpt1b [34]. Administration of L-selenomethione improved ammonia-mediated cardiac damage, indicating a role for methionine in maintaining cardiac function. In a cohort of hypertensive patients, hyperhomocysteinemia was increased in patients with cardiac hypertrophy, and the same study demonstrated that mice with induced hyperhomocysteinemia via methionine feeding in the diet had augmented cardiac hypertrophy after angiotensin II infusion [35]. Taken together with findings from the current study, dysregulated methionine metabolism in the heart may contribute to adverse cardiac remodeling.
References
- Sanghavi M, Rutherford JD. Cardiovascular physiology of pregnancy. Circulation. 2014; 130: 1003-1008.
- Umar S, Nadadur R, Iorga A, Amjedi M, Matori H, et al. Cardiac structural and hemodynamic changes associated with physiological heart hypertrophy of pregnancy are reversed postpartum. J Appl Physiol (1985). 2012; 113: 1253-1259.
- Chung E, Yeung F, Leinwand LA. Akt and MAPK signaling mediate pregnancy-induced cardiac adaptation. J Appl Physiol (1985). 2012; 112: 1564-1575.
- McMullen JR, Jennings GL. Differences between pathological and physiological cardiac hypertrophy: novel therapeutic strategies to treat heart failure. Clin Exp Pharmacol Physiol. 2007; 34: 255-262.
- Creanga AA, Syverson C, Seed K, Callaghan WM. Pregnancy-Related Mortality in the United States, 2011-2013. Obstet Gynecol. 2017; 130: 366-373.
- Wilson PW, D’Agostino RB, Sullivan L, Parise H, Kannel WB. Overweight and obesity as determinants of cardiovascular risk: the Framingham experience. Arch Intern Med. 2002; 162: 1867-1872.
- Brown DW, Giles WH, Croft JB. Left ventricular hypertrophy as a predictor of coronary heart disease mortality and the effect of hypertension. Am Heart J. 2000; 140: 848-856.
- Woodiwiss AJ, Libhaber CD, Majane OH, Libhaber E, et al. Obesity promotes left ventricular concentric rather than eccentric geometric remodeling and hypertrophy independent of blood pressure. Am J Hypertens. 2008; 21: 1144-1151.
- Chu SY, Bachman DJ, Callaghan WM, Whitlock EP, Dietz PM, et al. Association between obesity during pregnancy and increased use of health care. N Engl J Med. 2008; 358: 1444-1453.
- Abdullah A, Hoq S, Choudhary R, Laifer S, Zarich S. Cardiac performance is impaired in morbidly obese pregnant females. J Obstet Gynaecol Res. 2012; 38: 258-265.
- Buddeberg BS, Sharma R, O’Driscoll JM, Kaelin Agten A, Khalil A, et al. Cardiac maladaptation in obese pregnant women at term. Ultrasound Obstet Gynecol. 2019; 54: 344-349.
- Dudick K, Che C, Shoemaker R. Differential cardiac geometry during pregnancy in lean versus obese mice. Rev Cardiovasc Med. 2022; 23: 40.
- Che C, Dudick K, Shoemaker R. Cardiac hypertrophy with obesity is augmented after pregnancy in C57BL/6 mice. Biol Sex Differ. 2019; 10: 59.
- Yang Y, Kurian J, Schena G, Johnson J, Kubo H, et al. Cardiac Remodeling During 326 Pregnancy With Metabolic Syndrome: Prologue of Pathological Remodeling. Circulation. 2021; 143: 699-712.
- Fukushima A, Lopaschuk GD. Cardiac fatty acid oxidation in heart failure associated with obesity and diabetes. Biochim Biophys Acta. 2016; 1861: 1525-1534.
- Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, et al. Heart Disease and Stroke 331 Statistics-2017 Update: A Report From the American Heart Association. Circulation. 2017; 135: :e146-332 e603.
- Geiss GK, Bumgarner RE, Birditt B, Dahl T, Dowidar N, et al. Direct multiplexed measurement of gene expression with color-coded probe pairs. Nat Biotechnol. 2008; 26: 317-325.
- Hales CM, Carroll MD, Fryar CD, Ogden CL. Prevalence of Obesity Among Adults and Youth: United States, 2015-2016. NCHS Data Brief. 2017; 288: 1-8.
- Rich-Edwards JW, McElrath TF, Karumanchi SA, Seely EW. Breathing life into the lifecourse approach: pregnancy history and cardiovascular disease in women. Hypertension. 2010; 56: 331-334.
- Sundstrom J, Lind L, Vessby B, Andren B, Aro A, et al. Dyslipidemia and an unfavorable fatty acid profile predict left ventricular hypertrophy 20 years later. Circulation. 2001; 103: 836-841.
- Melchiorre K, Sharma R, Khalil A, Thilaganathan B. Maternal Cardiovascular Function in Normal 345 Pregnancy: Evidence of Maladaptation to Chronic Volume Overload. Hypertension. 2016; 67: 754-762.
- Abel ED, Litwin SE, Sweeney G. Cardiac remodeling in obesity. Physiol Rev. 2008; 88: 389-419.
- Tadinada SM, Weatherford ET, Collins GV, Bhardwaj G, Cochran J, et al. Functional resilience of C57BL/6J mouse heart to dietary fat overload. Am J Physiol Heart Circ Physiol. 2021; 321: H850-h64.
- Houten SM, Denis S, Argmann CA, Jia Y, Ferdinandusse S, et al. Peroxisomal L-bifunctional enzyme (Ehhadh) is essential for the production of medium-chain dicarboxylic acids. J Lipid Res. 2012; 53: 1296-1303.
- Chen MC, Chang JP, Lin YS, Pan KL, Ho WC, et al. Deciphering the gene expression profile of 356 peroxisome proliferator-activated receptor signaling pathway in the left atria of patients with mitral regurgitation. J Transl Med. 2016; 14: 157.
- Westin MA, Hunt MC, Alexson SE. The identification of a succinyl-CoA thioesterase suggests a novel 359 pathway for succinate production in peroxisomes. J Biol Chem. 2005; 280: 38125-38132.
- Shimojo N, Miyauchi T, Iemitsu M, Irukayama-Tomobe Y, Maeda S, et al. Effects of medium-chain triglyceride (MCT) application to SHR on cardiac function, hypertrophy and expression of endothelin-1 mRNA and other genes. J Cardiovasc Pharmacol. 2004; 44: S181-5.
- Iemitsu M, Shimojo N, Maeda S, Irukayama-Tomobe Y, Sakai S, et al. The benefit of medium-chain triglyceride therapy on the cardiac function of SHRs is associated with a reversal of 367 metabolic and signaling alterations. Am J Physiol Heart Circ Physiol. 2008; 295: H136-44.
- Saifudeen I, Subhadra L, Konnottil R, Nair RR. Metabolic Modulation by Medium-Chain Triglycerides Reduces Oxidative Stress and Ameliorates CD36-Mediated Cardiac Remodeling in Spontaneously Hypertensive Rat in the Initial and Established Stages of Hypertrophy. J Card Fail. 2017; 23: 240-251.
- Yang H, Liu W, Song S, Bai L, Nie Y, et al. Proteogenomics Integrating Reveal a Complex 374 Network, Alternative Splicing, Hub Genes Regulating Heart Maturation. Genes (Basel). 2022;13:2; 375 31.
- Cox EJ, Marsh SA. A systematic review of fetal genes as biomarkers of cardiac hypertrophy in rodent models of diabetes. PLoS One. 2014; 9: e92903.
- Qi L, Qi Q, Prudente S, Mendonca C, Andreozzi F, et al. Association between a genetic variant related to glutamic acid metabolism and coronary heart disease in individuals with type 2 380 diabetes. JAMA. 2013; 310: 821-828.
- Xie H, Zha E, Zhang Y. Identification of Featured Metabolism-Related Genes in Patients with Acute Myocardial Infarction. Dis Markers. 2020; 2020: 8880004.
- Zhang X, Wang A, Wang X, Zhao Q, Xing H. Evaluation of L-Selenomethionine on Ameliorating 384 Cardiac Injury Induced by Environmental Ammonia. Biol Trace Elem Res. 2022; 200: 4712-47125.
- Deng Y, Li Z, An X, Fan R, Wang Y, et al. Hyperhomocysteinemia Promotes Cardiac Hypertrophy in Hypertension. Oxid Med Cell Longev. 2022; 2022: 1486157.