Case Report
Austin J Clin Case Rep. 2014;1(1): 1003.
Central Retinal Vein Occlusion in Acute Promyelocytic Leukemia and Heterozygosis’ for Factor V Leiden and Methylentetrahydrofolate Reductase (MTHFR) Gene Mutations
Natasa Colovic1,2,*, Nada Suvajdzic -Vukovic1,2, Natalija Kosanovic-Jakovic1,3, Predrag Miljic1,2, Irena Djunic2, Ana Vidovic1,2 and Dragica Tomin1,2
1Department of Medicine, University of Belgrade, Belgrade, Serbia
2Department of Hematology, Clinical Center for Serbia, Belgrade, Serbia
3Department of Clinic for Eye disease/medical retinal CCS, Serbia
*Corresponding author: Natasa Colovic, Department of Medicine, University Belgrade, Dr. Subotica 8, 11010 Belgrade, Serbia.
Received: April 15, 2014; Accepted: May 02, 2014; Published: May 09, 2014
Abstract
We present a 45-year-old man with low-risk acute promyelocytic leukemia in whom thrombosis of central retinal vein occurred a year after establishing diagnosis, during molecular remission while being on maintenance therapy with all-trans-retinoic acid, purinethol and methotrexate. As major risk factors leading to central retinal vein occlusion (CRVO) we identified heterozygosity for factor V Leiden (G1691A) and methylentetrahydrofolate reductase (C677T) gene mutations. Moreover, hyperhomocysteinemia of moderate degree, low plasma folate level and arterial hypertension appeared contributory factors. Hyperhomocysteinemia could be attributed to both methylentetrahydrofolate reductase (C677T) gene mutation and methotrexate administration. Screenings for inherited and acquired thrombophilia are mandatory for all patients with unusual thrombotic events such as CRVO whether they have history of leukemia or not.
Keywords: Central Retinal Vein Occlusion; Acute Promyelocytic Leukemia; All-Trans-Retinoic Acid; Factor V Leiden Mutation; MTHFR C677 Mutation
Abbreviations
APL: Acute Promyelocytic Leukemia; APTT: Activated Partial Thromboplastin Time; ATRA: All Trans Retinoic Acid; CRVO: Central Retinal Vein Occlusion; KCT: Kaolin Clotting Time; MTHFR: Methylentetrahydrofolate Reductase; PCR-RFLP: Polymerase- Chain-Reaction-Restriction Fragment Lenght Polymorphism; WBC: White Blood Cells
Introduction
Thrombosis may be a presenting manifestation at diagnosis in a significant proportion of patients with acute promyelocytic leukemia (APL) (up to 9.6%). However, a similar rate of thrombosis can occur during treatment of APL [1-5]. A major cause of thrombosis in APL is hypercoagubility typical of the disease itself and probably enhanced with all-transretinoic treatment [1,3-5].
Case Report
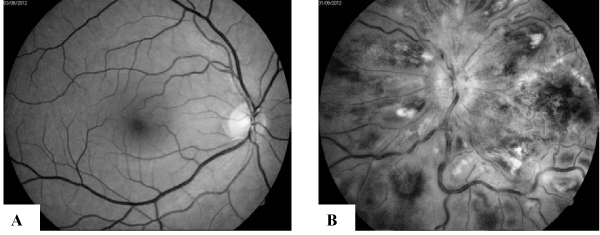
A 45-year-old man was diagnosed with APL in December 2010. His past medical history was significant only for arterial hypertension of 6-year duration. Both family and personal history of thrombosis were negative. Physical examination at admission was unremarkable except arterial tension of 160/100 mm Hg. A full blood count showed a white blood cell count (WBC) of 1.3x109/l, hemoglobin of 135 g/l and platelets of 165x109/l. There were 10% of atypical promyelocytes and blasts in peripheral blood. Blasts and promyelocytes comprising 44% of nucleated cells in a bone marrow aspirate were strongly myeloperoxidase positive. The immunophenotype of bone marrow blast cells was as follows: (alfaMPO, CD117, CD33, CD13, CD2)+ and (HLA-DR, CD34)-. Cytogenetic analysis of bone marrow cells revealed 46XY,t(15;17)(q22:q11-21)[5]/46,XY[25]. PML/RARA fusion transcript was of bcr1 type. The patient was diagnosed as low-risk APL. Coagulopathy was present with D-dimer 701µg/L (normal: < 240 ¯g/L ), prothrombin time (PT) 69% (normal: 75-120%), activated partial thromboplastin time (APTT) 27 sec (normal: 25-35 sec) and fibrinogen 2.41 g/l (normal: 2-4 g/L). The patient was treated according to the PETHEMA LPA 99 protocol (4). Prophilactic tranexamic acid was not administered in induction phase according to the reccomendations (4). He achieved cytologic and cytogenetic remission after induction treatment. Furthermore, he received three courses of consolidation chemotherapy according to the same protocol achieving molecular remission. In May 2011 he was started on the maintenance therapy with all-trans- retinoicacid (ATRA), purinethol and methotrexate. However, in January 2012 the patient exhibited a decrease in visual acuity progressing to severe loss of vision on left eye after a week interval. Ophtalmological examination performed 7 days after the onset of visual deterioration detected ischemic central retinal vein occlusion (CRVO) in the left eye; the finding in the right eye was normal (Figure 1).
Figure 1 :Fundus photography of the of the right eye: A. normal finding; B. left eye - disc swelling with blurred disc margins, dilated tortuous and engorged veins, confluent intaretinal haemorrhages, “cotton wool” exudates and retinal oedema.
Detailed hematologic work-up showed that the patient was still in molecular remission of his APL. Screening tests for thrombophilia have been done: fibrinogen 5,28 g/l, PT 89%, APTT 26 sec, kaolin clotting time (KCT) 94,1s (normal: 70-90 s), lupus anticoagulant (LA), 41,3s (normal: < 45s), Antithrombin 130% (normal: 80-120%), protein C (PC) 142 % (normal: 70-140%), APCR 1,7 (normal: 2,1- 3,5), Protein S 104% (normal: 63-135%). Anticardiolopin antibodies were in the normal ranges. In addition, plasma IgG and IgM, CRP, total protein, plasma triglicerides, cholesterol, rheumatod factor, and antinucelar antibodies were in normal ranges. However, plasma homocysteine level was moderately increased – 15.3 umol/L (normal: < 10) while plasma folate concentration was 5.3 ng/dL (normal: 5-16 ng/ml). By polymerase-chain-reaction-restriction fragment lenght polymorphism (PCR-RFLP) method it was found that the patient was a compound heterozygous carrier for Factor V Leiden mutation (G1691A) and 5,10-methylentetrahydrofolate reductase MTHFR gene (C677T). No mutations in genes coding prothrombin (G20210A) and PAI-1 (4G/5G) were detected.
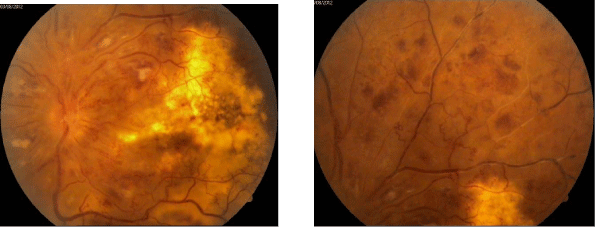
The patient was treated with anticoagulans together with antiglaucoma therapy (dorzolamide/timolol eye drops with no improvement in his vision. Although in August 2012 neovascular glaucoma developed (Figure 2).
Figure 2 :Fundus photography of the the left eye - posterior pole and upper part of the retinal periphery. Severe diffuse retinal ishaemia with vascular obliteration.
Subsequently, panretinal laserphotocoagulation was initiated but the patient remained completely blind in his left eye with increased intraocular pressure of 35 mmHg. Concerning APL, he has been still in complete molecular remission 27 months after diagnosis, pursuing maintenance chemotherapy.
Discussion
APL, a subtype of AML is characterized by life-threating bleeding and occasionally with thromboembolic events occuring typically during induction therapy with ATRA and idarubicin [4-9]. Conversely, occurence of arterial and venous thromboses, including CRVO throughout complete remission of APL are much rarer and should be analyzed in view of other usually nonleukemic prothrombotic contributory factors. Therefore, CRVO may beassociated with a number of local and systemic risk factors such as arterial hypertension diagnosed in 25% of patients, inflammation, malignancy, arteriosclerosis, congenital anomalies of CRV, diabetes, high level of low density lipoproteins, migraine and chronic glaucoma, hyperhomocysteinemia and advanced age [8-11]. Besides, the infections which are common during remission induction chemotherapy may produce endothelial cell damage followed by cytokine activation and consequent venous or arterial thrombosis. Nevertheless, the major contributory prothrombotic factors in CRVO, especially younger adults are hereditary thrombophilia among which the most frequent is factor V Leiden mutation (G1691A) [10]. In addition, C677T single nucleotide polymorphism in the MTHFR gene leading to moderate hyperhomocysteinemia has been reported as a significant risk factor for CRVO [12-15]. Besides, hyperhomocysteinemia in our patient could be caused by low folatemia as a consequence of methotrexate administration. Hyperhomocysteinemia causes both oxidative damage to vascular endothelial cells and increased proliferation of vascular smooth muscle cells [16] and is recognized in several recent studies as an independent risk factor for CRVO. Concerning this issue, our patient exhibited several risk factors for CRVO such as arterial hypertension, two inherited thrombophilic factors, hyperhomocysteinemia and low folate levels. In addition, we cannot rule out the role of methotrexate administration as a trigger/contributory factor for CRVO in our patient via mechanism of hyperhomocysteinemia. Addressing this issue it would be of interest to investigate prospectively the rate of thromboembolic events during maintenance phase of APL.
Conclusion
In all patients with acute leukemia and unusual thrombotic events such as CRVO, screening tests for inherited and acquired thrombophilia are mandatory whether they have history of leukemia or not.
Conflicts of Interest
There is no Conflict of Interest inside this manuscript.
This case report has been approved by an ethics institutional review board.
References
- Breccia M, Avvisati G, Latagliata R, Carmosino I, Guarini A, De Propris MS, et al. Occurrence of thrombotic events in acute promyelocytic leukemia correlates with consistent immunophenotypic and molecular features. Leukemia. 2007; 21: 79–83.
- De Stefano V, Sora F, Rossi E, Chiusolo P, Laurenti L, Fianchi L et al. The risk of thrombosis in patients with acute leukemia:occurence of thrombosis at diagnosis and during treatment. J Thromb Haemost. 2005; 3: 1985-1992.
- Ziegler S, Sperr WR, Knöbl P, Lehr S, Weltermann A, Jäger U, et al. Symptomatic venous thromboembolism in acute leukemia. Incidence, risk factors, and impact on prognosis. Thromb Res. 2005; 115: 59-64.
- Choudhry A, DeLoughery TG. Bleeding and thrombosis in acute promyelocytic leukemia. Am J Hematol. 2012; 87: 596-603.
- Falanga A, Russo L, Tartari CJ. Pathogenesis and treatment of thrombohemorrhagic diathesis in acute promyelocytic leukemia. Mediterr J Hematol Infect Dis. 2011; 3: e2011068.
- Sanz MA, Lo Coco F, Martín G, Avvisati G, Rayón C, Barbui T, et al. Definition of relapse risk and role of nonanthracycline drugs for consolidation in patients with acute promyelocytic leukemia: a joint study of the PETHEMA and GIMEMA cooperative groups. Blood. 2000; 96: 1247-1253.
- Sanz MA, Montesinos P. Open issues on bleeding and thrombosis in acute promyelocytic leukemia. Thromb Res. 2010; 125 Suppl 2: S51-54.
- Yates JW. Case management: acute promyelocytic leukemia. Clinical Oncology Alert. 2010; 5: 33-34.
- Prisco D, Marcucci R, Bertini L, Gori AM. Cardiovascular and thrombophilic risk factors for central retinal vein occlusion. Eur J Intern Med. 2002; 13: 163-169.
- Laroche L, Saraux H. Unilateral central retinal vein occlusion in systemic lupus erythematosus. Ophthalmologica. 1984; 189: 128-129.
- Walters RF, Spalton DJ. Central retinal vein occlusion in people aged 40 years or less: a review of 17 patients. Br J Ophthalmol. 1990; 74: 30-35.
- Larsson J, Olafsdottir E, Bauer B. Activated protein C resistance in young adults with central retinal vein occlusion. Br J Ophthalmol. 1996; 80: 200-202.
- Jaksic V, Markovic V, Milenkovic S, Stefanovic I, Jakovic N, Knezevic M. MTHFR C677T homozygous mutation in a patient with pigmentary glaucoma and central retinal vein occlusion. Ophthalmic Res. 2010; 43: 193-196.
- Marcucci R, Bertini L, Giusti B, Brunelli T, Fedi S, Cellai AP, et al. Thrombophilic risk factors in patients with central retinal vein occlusion. Thromb Haemost. 2001; 86: 772-776.
- Pianka P, Almog Y, Man O, Goldstein M, Sela BA, Loewenstein A. Hyperhomocysteinemia in patients with nonarteric anterior ischemic optic neuropathy, central retinal artery occlusion, and central vein occlusion. Ophtalmology. 2000; 107:1588-1592.
- Li M, Chen J, Li YS, Feng YB, Gu X, Shi CZ. Folic acid reduces adhesion molecules VCAM-1 expession in aortic of rats with hyperhomocysteinemia. Int J Cardiol. 2006; 106: 285-288.
Citation: Colovic N, Suvajdzic-Vukovic N, Kosanovic-Jakovic N, Miljic P, Djunic I, et al. Central Retinal Vein Occlusion in Acute Promyelocytic Leukemia and Heterozygosis’ for Factor V Leiden and Methylentetrahydrofolate Reductase (MTHFR) Gene Mutations. Austin J Clin Case Rep. 2014;1(1): 1003. ISSN 2381-912X