Case Report
Austin J Clin Case Rep. 2014;1(4): 1016.
Febrile Illness Unmasks Brugada Syndrome
Aanchal Gupta* and Stephan L Kamholz
Department of Medicine, Albert Einstein College of Medicine, USA
*Corresponding author: Aanchal Gupta, Department of Medicine, Albert Einstein College of Medicine, 1576 Tomlinson Avenue, Bronx, , New York, 10461, USA
Received: May 21, 2014; Accepted: June 16, 2014; Published: June 18, 2014
Abstract
The Brugada syndrome is characterized by right bundle-branch block pattern on Electrocardiogram (ECG), right precordial ST-segment elevation [usually leads V1/V2] and syncope/sudden cardiac death in absence of significant electrolyte abnormalities, ischemia or structural heart disease [1]. A 59 year old presented with dizziness and fever due to pyogenic liver abscess. Multiple ECG’s during the hospital admission revealed a Brugada type 1 pattern. The ECG reverted to normal when fever resolved. Mutations in the cardiac sodium-channel gene SCN5A which cause a decrease in the inward depolarizing sodium current have been associated with the Brugada syndrome [2-4]. The dysfunction of the sodium channel is exaggerated at higher than physiologic temperatures; the syndrome may be unmasked by fever increasing the risk of ventricular arrhythmias and/or sudden cardiac death [5-8]. The association of febrile illness with the potential for life-threatening cardiac events, heralded by unique electrocardiographic abnormalities, should be recognized.
Keywords: Brugada syndrome; Fever; Ventricular fibrillation
Clinical Presentation
A 59 year-old man presented complaining of right-sided neck pain, fever and chills of one day’s duration. The patient also reported pain along the right shoulder blade and ipsilateral pleuritic chest pain, headache, and dizziness without any loss of consciousness. There was no past history of syncope/loss of consciousness or of nocturnal agonal respiration. His family history was unremarkable for syncope or sudden cardiac death. He had emigrated to the U.S from China many years ago.
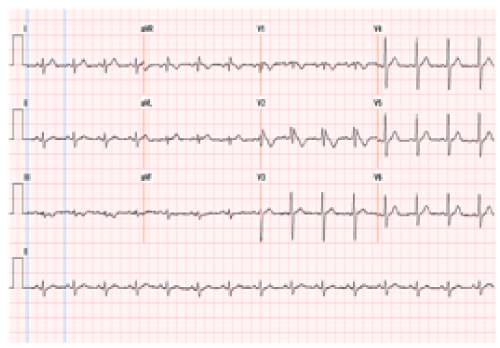
In the Emergency Department, fever was noted (40.2°C), with tachycardia (108 beats/min) and bibasilar rales on lung examination. The chest radiograph revealed mild decrease in bibasilar aeration with some elevation of the right hemi-diaphragm. There was no radiologic evidence of consolidation, pleural effusion or pneumothorax. ECG showed coved-type ST elevation in leads V1, V2 with right bundle branch block (RBBB) - Brugada type 1 pattern (Figure 1a). Laboratory findings were significant for leukocytosis (23,300/μL with 14% band forms), mild elevation of liver function tests (Table 1) and normal troponin. Transthoracic echocardiogram revealed a normal left ventricular ejection fraction and with no regional wall motion abnormality.
Figure 1a :Normal Sinus rhythm with coved-type ST elevation in leads V1, V2 with RBBB- Brugada type 1 pattern when the patient was febrile.
Hospital
day
AST
ALT
ALP
Albumin
1
89
97
74
4.2
3
154
228
105
3.7
10
63
96
425
3.2
21
27
27
183
3.4
Table 1: Laboratory results of Liver function tests during hospital stay.
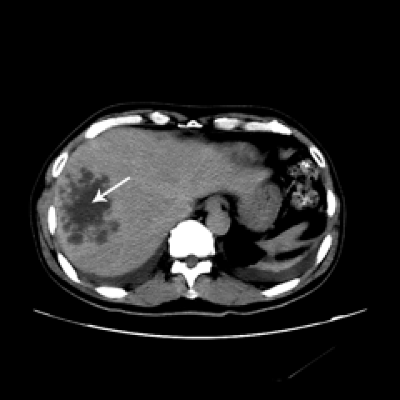
The patient was admitted to a telemetry unit for close monitoring of arrhythmias. The patient was persistently febrile despite initial empiric antibiotic treatment for community acquired pneumonia. Repeat ECG’s during the febrile course continued to demonstrate the Brugada type 1 pattern. Serial laboratory evaluation revealed worsening liver function tests (Table 1), and blood cultures were reported positive for Klebsiella pneumonia [On hospital day #7]. A Computerized Tomographic (CT) scan of the abdomen demonstrated a 9.0x 7.0 cm heterogeneous mass suggestive of a pyogenic liver abscess (Figure 2). CT guided aspiration of the liver lesion yielded 70 mL of purulent fluid, which was positive for Klebsiella pneumoniae on culture. Therapy with ceftriaxone and metronidazole was substituted for the initial antibiotic regimen.
Figure 2 :An image of the computerized tomograph of abdomen and pelvis with intravenous contrast showing a liver lesion most consistent with infection, likely pyogenic. No evidence of hemangioma or vascular neoplasm.
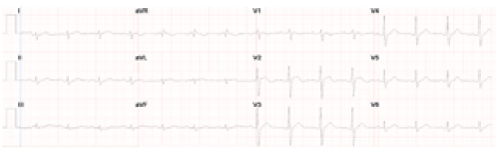
There were no documented episodes of arrhythmia on telemetry. However, the ECG immediately prior to hospital discharge (when the patient was afebrile) did not reveal the Brugada pattern (Figure 1b). The patient was discharged to complete a four-week course of specific antimicrobial therapy, and was scheduled for an electro-physiologic study to evaluate inducibility of ventricular arrhythmias.
Figure 3 :Normal Sinus rhythm, indeterminate axis, Non specific anterior T wave changes obtained when the patient fever resolved.
Discussion
Previous reports document that the Brugada ECG pattern [and concomitantly, the risk for lethal ventricular arrhythmias] may be unmasked by fever, regardless of the etiology of fever [5-8]. To our knowledge, this is the first report of the Brugada pattern being unmasked by fever induced by pyogenic liver abscess. It is important to recognize that this phenomenon, though rare, poses the potential for life threatening complications such as ventricular arrhythmias leading to sudden cardiac death.
Three different patterns of ST elevation have been described [9,10]. In the classic Brugada type 1 ECG, the elevated ST segment (≥2 mm) descends with an upward convexity to an inverted T wave. This is referred to as the ‘coved type’ Brugada pattern. The type 2 and type 3 patterns have a ‘saddle back’ ST-T wave configuration, in which the elevated ST segment (elevated ≥1 mm in type 2 and <1 mm in type 3) descends toward the baseline, and then rises again to an upright or biphasic T wave. The type 2 and 3 patterns are not diagnostic of Brugada syndrome. These three patterns may be observed spontaneously in serial ECG tracings from the same patient or after the administration of specific drugs [sodium channel blockers, vagotonic agents, alpha-adrenergic agonists, beta-adrenergic blockers, tricyclic or tetracyclic antidepressants].
The consensus report from the Study Group on the Molecular Basis of Arrhythmias of the European Society of Cardiology [10]; suggests that the Brugada syndrome should be considered in a patient if patient has classic Brugada pattern in more than one right precordial lead (V1 - V3), along with at least one of the other criteria which include documented ventricular fibrillation (VF), self-terminating polymorphic ventricular tachycardia (VT), family history of sudden cardiac death at <45 years, type 1 ST segment elevation in family members, electro-physiologic inducibility of ventricular tachycardia, unexplained syncope suggestive of a tachyarrhythmia, or nocturnal agonal respiration . The absence of these clinical findings suggested that it would be safe for the patient to have an ambulatory cardiac electro-physiologic study.
Although a diagnosis of Brugada syndrome can be made by typical ECG findings, sometimes these findings may only be unmasked in presence of certain physiologic stressors such as elevated body temperature [5-8]. Dumaine et al demonstrated that threonine in SCN5A gene; is an important determinant of the temperature sensitivity of the human cardiac sodium channel. A missense mutation in the same channel causes faster decay of inward sodium current leading to unopposed outward current during phase 1 of cardiac muscle action potential, accentuating the latter [5]. This phenomenon is observed in cells and tissues displaying a prominent outward current like the right ventricular epicardium. This creates a transmural voltage gradient and development of a large transmural dispersion of repolarization; phase 2 re-entry capable of precipitating a rapid polymorphic VT/VF responsible for sudden death in the Brugada syndrome [11].
The ability to detect these changes on ECG increases with placement of right precordial leads in higher intercostal spaces [12]. In this patient, the initial ECG obtained in the Emergency Department had an isolated 3mm ST segment elevation in V2. However, repeat ECG with higher right precordial leads revealed Brugada type 1 pattern in V1, V2. This emphasizes the importance of obtaining multiple ECG’s (including a 12 lead ECG with high right precordial leads), if there is any prior history of syncope/dizziness during a febrile illness or during the current illness, thus facilitating the identification of a Brugada-like pattern unmasked by fever.
We suggest that it is reasonable to obtain an ECG in patients (both young and old) presenting with a febrile illness, in the absence of prior cardiac history. Multiple studies have shown that fever (independent of its etiology), induces arrhythmogenicity in the SCN5A gene [13]. Obtaining an ECG and aggressively controlling fever with antipyretics could prevent potentially lethal ventricular arrhythmias.
References
- Brugada P, Brugada J. Right bundle branch block, persistent ST segment elevation and sudden cardiac death: a distinct clinical and electrocardiographic syndrome. A multicenter report. J Am Coll Cardiol. 1992; 20: 1391-1396.
- Kapplinger JD, Tester DJ, Alders M, Benito B, Berthet M, Brugada J, et al. An international compendium of mutations in the SCN5A-encoded cardiac sodium channel in patients referred for Brugada syndrome genetic testing. Heart Rhythm. 2010; 7: 33-46.
- Amin AS, Asghari-Roodsari A, Tan HL. Cardiac sodium channelopathies. Pflugers Arch. 2010; 460: 223-237.
- Liang P, Liu W, Li C, Tao W, Li L, Hu D. Genetic analysis of Brugada syndrome and congenital long-QT syndrome type 3 in the Chinese. J Cardiovasc Dis Res. 2010; 1: 69-74.
- Dumaine R, Towbin JA, Brugada P, Vatta M, Nesterenko DV, Nesterenko VV, et al. Ionic mechanisms responsible for the electrocardiographic phenotype of the Brugada syndrome are temperature dependent. Circ Res. 1999; 85: 803-809.
- Mok NS, Priori SG, Napolitano C, Chan NY, Chahine M, Baroudi G. A newly characterized SCN5A mutation underlying Brugada syndrome unmasked by hyperthermia. J Cardiovasc Electrophysiol. 2003; 14: 407-411.
- Keller DI, Rougier JS, Kucera JP, Benammar N, Fressart V, Guicheney P, et al. Brugada syndrome and fever: genetic and molecular characterization of patients carrying SCN5A mutations. Cardiovasc Res. 2005; 67: 510-519.
- Kurisu S, Inoue I, Kawagoe T, Ishihara M, Shimatani Y, Nakama Y, et al. Therapeutic hypothermia after out-of-hospital cardiac arrest due to Brugada syndrome. Resuscitation. 2008; 79: 332-335.
- Antzelevitch C, Brugada P, Borggrefe M, Brugada J, Brugada R, Corrado D, et al. Brugada syndrome: report of the second consensus conference: endorsed by the Heart Rhythm Society and the European Heart Rhythm Association. Circulation. 2005; 111: 659-670.
- Wilde AA, Antzelevitch C, Borggrefe M, Brugada J, Brugada R, Brugada P, et al. Proposed diagnostic criteria for the Brugada syndrome. Eur Heart J. 2002; 23: 1648-1654.
- Antzelevitch C, Belardinelli L. The role of sodium channel current in modulating transmural dispersion of repolarization and arrhythmogenesis. J Cardiovasc Electrophysiol. 2006; 17: S79-79S85.
- wataru shimizu, kiyotaka matsuo , masahiko takagi, yasuko tanabe, takesi aiba, atsushi taguchi, et al. Body surface distribution and response to drugs of ST segment elevation in Brugada syndrome: clinical implication of eighty-seven-lead body surface potential mapping and its application to twelve-lead electrocardiograms. J Cardiovasc Electrophysiol. 2000; 11: 396-404.
- Amin AS, Meregalli PG, Bardai A, Wilde AA, Tan HL. Fever increases the risk for cardiac arrest in the Brugada syndrome. Ann Intern Med. 2008; 149: 216-218.