Case Report
Austin J Clin Case Rep. 2014;1(6): 1029.
Is it the Heart or the Brain?
Dashora Umesh1, Al-Abdullah Alhasan2* and Joarder Rita3
1Department of Medicine, Conquest Hospital, UK
2Department of Oncology, Guy’s Hospital, UK
3Department of Radiology Conquest Hospital, UK
*Corresponding author: Al-Abdullah Alhasan, Department of Oncology, Guy’s Hospital, UK
Received: June 28, 2014; Accepted: July 20, 2014; Published: July 24, 2014
Abstract
A 66 year old gentleman was brought to the Accident and Emergency department after he was found unconscious in the toilet. He had new ECG changes. An urgent CT scan showed no haemorrhage, infarction or space-occupying lesion. The patient was initially treated as a case of acute coronary syndrome. The troponin level 12 hours after the episode was normal. Consciousness gradually improved and further neurological examination revealed gait ataxia with right sided deviation. MRI scan of the brain showed a large acute cerebellar infarct. The patient improved with the standard treatment regimen for stroke and made a good recovery.
Keywords: ECG changes in acute stroke; Heart; Brain
Abbreviations
ECG: Electrocardiography; CT: Computerised Tomography; MRI: Magnetic Resonance Imaging; GCS: Glasgow Coma Scale; CRP: C - reactive protein; PICA: Posterior Inferior Cerebellar Artery; ACS: Acute Coronary Syndrome; INR: International Normalized Ratio
Case Presentation
A 66 year old gentleman presented with his partner to the Accident and Emergency department after he had been found unconscious in the toilet. According to his partner, he had complained of a headache, nausea and vomiting for two days. There were no such episodes in the past. There was a past history of myocardial infarction, pericarditis, and paroxysmal atrial fibrillation on warfarin treatment, hypertension and peripheral vascular disease. He had stopped all his medications 15 days before this episode. On examination, he was pale and drowsy with a Glasgow Coma Scale score of 7/15 on initial review by paramedical staff. Consciousness gradually improved with GCS score rising to 11/15 by the time he arrived to the hospital. Pulse was 63 per minute irregular and BP 176/86 mm of Hg. Chest, heart and abdominal examinations were unremarkable. Initial neurological examination revealed no nystagmus, normal eye movements, no facial droop, normal reflexes and tone. The patient’s GCS improved to 15/15 within 12 hours. He denied any chest pain in relation to this episode. More detailed neurological examination was possible after 12 hours and revealed gait ataxia with right sided deviation. Interestingly, there was no dysarthria, dysphagia, limb ataxia, dysmetria, past-pointing, dysdiadochokinesis or rebound phenomenon; nor could we find any positive signs of brain stem dysfunction including Horner’s syndrome, dissociated sensory loss, pathological head-impulse test or paresis of the soft palate.
Investigations
Blood tests showed normal blood counts, renal function and CRP. High sensitivity troponin on admission was 11ng/l with no change after 6 hours. The INR was 1.0.
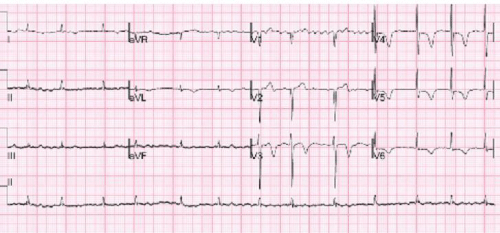
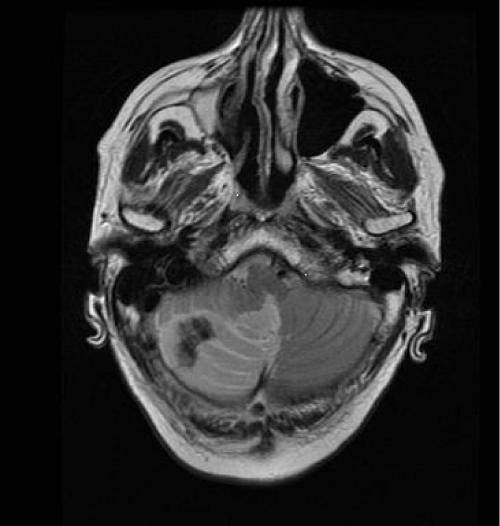
An ECG on admission showed flutter fibrillation, ST segment flattening in leads v1-v3 with T wave inversion in multiple leads (changes were new and dynamic compared to previous ECGs) (Figure1). In view of the altered consciousness level and sub-therapeutic INR, an urgent CT brain was performed on admission which showed no acute infarction or bleed and no space-occupying lesion. When ataxia was noted on the second day, an urgent MRI scan of the brain was requested. The scan could only be performed 5 days after admission and showed a large acute right sided ischaemic infarction with marked associated oedema, midline shift of cerebellum and distortion of the brainstem and fourth ventricle (Figure 2).
Figure 1 : ECG showing atrial flutter-fibrillation with new T wave inversion in multiple leads.
Figure 2 : MRI showing right sided cerebellar infarction with gross oedema, midline shift and distortion of the brainstem and fourth ventricle. There was no evidence of hemorrhage on the T1 sequences of the MRI or on the recent CT.
There was no pathological enhancement after intravenous gadolinium.
Differential Diagnosis
Clinical features like sudden onset collapse in association with new ECG changes of deep symmetrical T wave changes would favour the diagnosis of acute coronary syndrome or a non ST elevation myocardial infarction. These conditions would require anticoagulation. On the other hand, it is important to exclude a haemorrhagic stroke in view of sudden onset unconsciousness with preceding headache and vomiting. Anticoagulation would be detrimental in cases of haemorrhagic stroke and a totally different treatment would be needed in ischaemic stroke. Finally it is also possible for a patient with atrial fibrillation to have simultaneous embolism of right PICA and coronary system.
Treatment
This patient was initially treated with ACS protocol (Aspirin, Clopidogrel and Low molecular weight heparin). The treatment was changed to Aspirin 300 mg for 2 weeks as soon as gait ataxia was noted on neurological assessment. Following the MRI scan a neurosurgical opinion was sought. A conservative approach with medical treatment was advised. His other treatment included dipyridamol 200 mg BD, atorvastatin 80 mg daily, perindopril 2 mg daily, bisoprolol 1.25 mg daily, amlodipine 10 mg daily, digoxin 25 μg daily and GTN spray. The patient responded well to the treatment and was able to go home after a few days. Oral anticoagulation was resumed after two weeks.
Outcome and Follow up
The patient managed a full recovery and had no residual neurological deficit.
A cardiac CT angiogram was performed after two months in view of his ECG changes. This was suggestive of significant stenosis in the proximal right coronary artery but no evidence of any significant plaque or stenosis in left main or left anterior descending artery. The patient remained asymptomatic and chose not to have a formal angiogram.
Discussion
The posterior inferior cerebellar artery (PICA) supplies blood to the vermis and lateral medulla. A complete PICA artery infarct is usually associated with mass effect and leads to headache, vomiting, ataxia and gaze palsies. The patients keep their head tilted to the side of lesion. A PICA infarct involving only the lateral side causes dizziness and gait in-coordination with lateral reeling to the side of lesion and there may be limb ataxia. Medial PICA infarction causes vertigo and nystagmus and some patients may feel pulling towards the side of lesion (lateropulsion) [1]. Large cerebellar infarcts are associated with oedema formation and mass effect and therefore are called acute psedotumoral cerebellar infarctions. When severe, this can lead to headache, drowsiness and ultimately coma with bilateral extensor plantar response [1]. Our patient was found unconscious after complaining of headache, nausea and vomiting (features in keeping with raised intracranial pressure). Following recovery of his GCS, neurological examination revealed gait ataxia with right sided deviation possibly because of lateral PICA infarct involving mainly the lateral side of cerebellum. The loss of consciousness was possibly a result of transient brain-stem dysfunction. Alternatively, it is possible that an arrhythmic cardiac event caused cardiac syncope and prompted cardiac embolism causing the stroke. Simultaneous embolism of right PICA and coronary system could also be a reasonable explanation of the clinical picture. The patient had a history of paroxysmal atrial fibrillation and the INR was sub-therapeutic on admission.
Unenhanced CT is the usual initial imaging test for acute stroke to exclude haemorrhage [2,3]. The imaging also helps to estimate the tissue at risk and excludes stroke mimics like tumours. CT is preferred over MRI because of widespread availability, rapid scanning time and ease of detecting haemorrhage. MR is useful in posterior circulation strokes. As seen in this case, appropriate MRI imaging is advantageous in very early ischaemic stroke (diffusion weighted imaging) and is also useful in detecting hyper acute haemorrhage. MR or CT angiogram is helpful in assessing the vascular tree and detecting intravascular thrombi and dissections.
In our patient with low GCS, paucity of brain stem and cerebellar neurological signs, normal CT scan and new ECG changes, the collapse was initially thought to be of cardiac origin. Subsequently ataxia was picked up and an MRI scan was requested. MRI showed a large acute cerebellar hemisphere infarction (Figure 2). In retrospect, unconsciousness at presentation should have prompted an alternative or additional diagnosis to ACS. Echocardiogram was not performed but might have helped by picking up or excluding any regional wall motion abnormalities or mural thrombus.
ECG was abnormal in 92 percent of patients with acute stroke in one study of 150 patients [4]. ECG changes in the form of classic large and upright T waves are well known in subarachnoid haemorrhage. Less commonly these changes are also seen in other strokes [4,5]. Other ECG abnormalities described in stroke are QT prolongation [4], Q wave changes, ST segment changes, T wave inversions, U waves and arrhythmia [4]. T wave inversion is four times more common in patients with stroke compared to age matched controls [5].
ECG changes in strokes are thought to be because of subendocardial ischaemia which is likely to be the result of sympathetic outflow due to hypothalamic ischaemia possibly due to raised intracranial pressure [4]. These abnormalities disappear after brain death suggesting neural origin. Moreover these patients also fail to reveal any cardiac abnormality at autopsy [6]. Additionally, in stroke, the normal circadian rhythm of heart rate and blood pressure is reversed for up to 6 months with increase in heart rate and blood pressure at night because of sympathetic dominance rather than vagal dominance. This may explain some of the arrhythmias seen without any structural abnormalities in the coronary arteries or heart [7]. A more detailed coverage of this subject can be found in Up-to-date [8].
ST segment changes in 5-11 percent might actually be due to co-existing myocardial infarction [9]. In older patients over 65 years and in patients with diabetes these changes are more likely to represent actual ischemia [9]. Q waves similar to those seen in myocardial infarction may also reflect a coexisting cardiac event [4].
The Troponin level can also rise after a stroke and does not automatically prove ACS unless indicated by the clinical picture [10].
Cerebellar infarctions in association with ECG changes have not been specifically described in the literature. In our patient, the ECG changes consisted of deep symmetric T wave inversion which reversed after a few days and hence were probably due to cerebellar stroke.
Cerebellar strokes can be associated with raised intracranial pressure and distortion of the brainstem with resultant risk to vital organs. Therefore a low threshold for intubation and early neurosurgical consultation is prudent. Once intracranial haemorrhage has been excluded, the acute management is similar to the management of acute ischaemic stroke. IV thrombolysis should have the same efficacy in cerebellar stroke as in anterior circulation infarction but the data is limited [11,12]. Fortunately, despite the delay in performing the MRI scan and significant changes on the scan, our patient improved with conservative medical treatment as described above.
References
- Caplan LR. Posterior circulation cerebrovascular syndromes. UpToDate 2012.
- Adams HP Jr, del Zoppo G, Alberts MJ, Bhatt DL, Brass L, Furlan A, et al. Guidelines for the early management of adults with ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council, Clinical Cardiology Council, Cardiovascular Radiology and Intervention Council, and the Atherosclerotic Peripheral Vascular Disease and Quality of Care Outcomes in Research Interdisciplinary Working Groups: the American Academy of Neurology affirms the value of this guideline as an educational tool for neurologists. Stroke. 2007; 38: 1655-1711.
- Latchaw RE, Alberts MJ, Lev MH, Connors JJ, Harbaugh RE, Higashida RT, et al. Recommendations for imaging of acute ischemic stroke: a scientific statement from the American Heart Association. Stroke. 2009; 40: 3646-3678.
- Goldstein DS. The electrocardiogram in stroke: relationship to pathophysiological type and comparison with prior tracings. Stroke. 1979; 10: 253-259.
- Dimant J, Grob D. Electrocardiographic changes and myocardial damage in patients with acute cerebrovascular accidents. Stroke. 1977; 8: 448-455.
- Yamour BJ, Sridharan MR, Rice JF, Flowers NC. Electrocardiographic changes in cerebrovascular hemorrhage. Am Heart J. 1980; 99: 294-300.
- Korpelainen JT, Sotaniemi KA, Huikuri HV, Myllylä VV. Circadian rhythm of heart rate variability is reversibly abolished in ischemic stroke. Stroke. 1997; 28: 2150-2154.
- Chalela JA, Jacobs TL. Cardiac complications of stroke. UpToDate. 2012.
- Lavy S, Yaar I, Melamed E, Stern S. The effect of acute stroke on cardiac functions as observed in an intensive stroke care unit. Stroke. 1974; 5: 775-780.
- Scheitz JF, Endres M, Mochmann HC, Audebert HJ, Nolte CH. Frequency, determinants and outcome of elevated troponin in acute ischemic stroke patients. Int J Cardiol. 2012; 157: 239-242.
- Furie KL, Kasner SE, Adams RJ, Albers GW, Bush RL, Fagan SC, et al. Guidelines for the prevention of stroke in patients with stroke or transient ischemic attack: a guideline for healthcare professionals from the american heart association/american stroke association. Stroke. 2011; 42: 227-276.
- Fiebach JB, Schellinger PD, Gass A, Kucinski T, Siebler M, Villringer A, et al. Stroke magnetic resonance imaging is accurate in hyperacute intracerebral hemorrhage: a multicenter study on the validity of stroke imaging. Stroke. 2004; 35: 502-506.