Case Report
Austin J Clin Case Rep. 2014;1(9): 1043.
Ovarian Rhabdomyosarcoma Arising from Mature Cystic Teratoma
Sanz-Baro R1*, Gonzalez Alvarez MC2, Fernández-Aceñero MJ3 and Di Fiore HA4
1Department of Obstetrics and Gynecology, Fundación Jiménez Díaz University Hospital, Spain
2Department of Obstetrics and Gynecology, Fundación Jiménez Díaz University Hospital, Spain
3Department of Pathology, Fundación Jiménez Díaz University Hospital, Spain
4Department of Obstetrics and Gynecology, Fundación Jiménez Díaz University Hospital, Spain
*Corresponding author: Sanz-Baro R, Department of Obstetrics and Gynecology, Fundación Jiménez Díaz University Hospital, Avda, Reyes Católicos, 2. 28040 Madrid, Spain
Received: August 01, 2014; Accepted: September 01, 2014; Published: September 03, 2014
Abstract
Malignant transformation of mature cystic teratoma is very unusual and has difficult diagnosis and poor outcome. We present the case of a young woman diagnosed with a benign teratoma measuring 15 cm, and a laparotomic adnexectomy was performed. Following diagnosis of rhabdomiosarcoma in mature cystic teratoma, she underwent complete cytoreductive surgery. Despite complete surgery and adjuvant therapy, the patient died a few months after the diagnosis. We report a rare case of malignant transformation of mature teratomas with a very poor outcome.
Keywords: Malignant transformation; Mature cystic teratoma; Ovary; Rhabdomiosarcoma
Abbreviations
CEA: Carcinoembryonic Antigen; CA: Carbohydrate Antigen; CT: Computed Tomography; AFP: Alpha Fetoprotein; VAC: Vincristine Actinomycine D Cyclophosphamide
Case Presentation
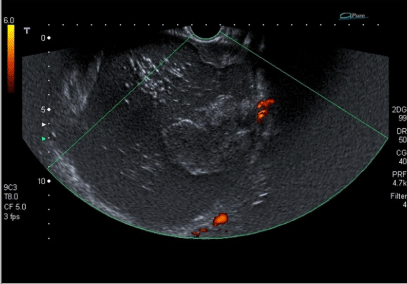
The patient was a 27 year-old nulliparous woman. She was treated at the emergency department in September 2011 with a pelvic mass and constipation. She was referred for gynecologic examination due to a large mobile pelvic mass that extended from almost to the umbilicus. An ultrasound examination showed a solid/cystic mass measuring 149x 127 mm containing hair and sebaceous material. None of the solid components had increased vascularization, and she was diagnosed with benign teratoma of the left ovary (Figure 1). No other imaging studies were performed, as diagnosis seemed clear. Preoperative chest X-Ray, laboratory tests, Pap smear, and tumor markers (CEA, Ca 125 and Ca 19.9) were normal.
Figure 1: Doppler ultrasound: solid-cystic mass, fatty component, hair, and no increased vascularisation.
Left adnexectomy by laparotomy was performed 20 days after the diagnosis revealing a mass measuring 18 cm and a normal uterus and contralateral ovary. There were no tumor masses in the pelvis or upper abdomen and the left ovarian capsule had not been ruptured.
The surgical specimen was a left salpingo-oophorectomy, weighing 360 g and comprising fallopian tube with a length of 7 cm length and a 17.5 x 15.5 x 5cm ovary. Serial sectioning of the ovary revealed a cystic mass, containing hair and abundant sebaceous yellowish material. In the cyst wall we identified a white whorled tumor with large necrotic foci measuring 8 cm. We also found a small serosal nodule in the tubal wall; which was then paraffin-embedded for histopathological analysis.
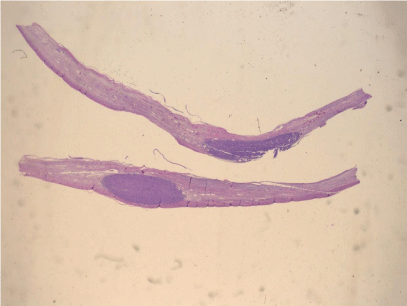
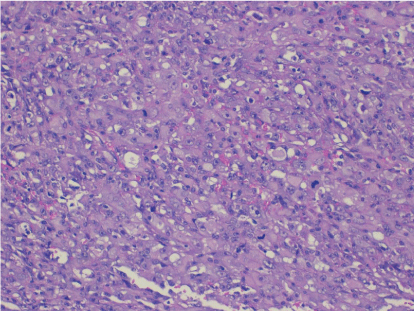
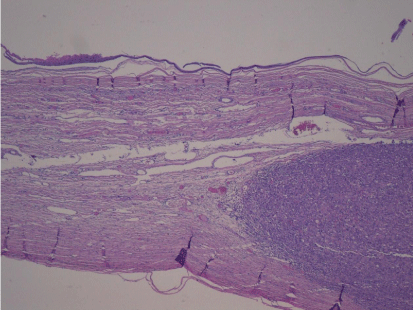
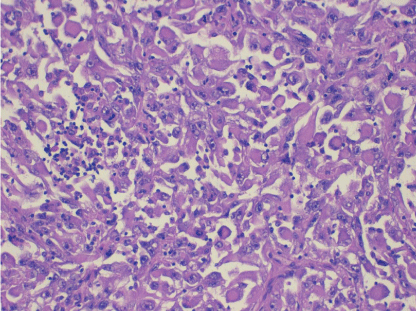
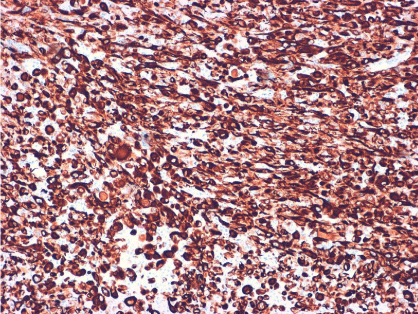
Pathological analysis revealed a mature dermoid cyst, with areas of epidermal differentiation in approximately 40% of the cyst wall (Figure 2a and 2b). Following histopathological analysis, the nodule found in the cyst wall was identified as a highly pleomorphic tumor with extensive necrosis and many atypical mitoses with irregular mitotic spindle assembly (Figure 3). Some areas showed rhabdomyoblasts (Figure 4). An immunohistochemistry study confirmed that the tumor cells were made up of muscle tissue and were strongly positive for desmin and myoglobin (Figure 5) and negative for epithelial markers (including wide spectrum cytokeratins and epithelial membrane antigen), therefore discarding a possible diagnosis of extrarenal rhabdoid tumor (all antibodies from Dako Corp., Denmark).
Figure 2a: Low power view of the cyst wall showing a mural densely cellular nodule, underneath the cyst lining (H-E x 4).
Figure 2b: Epidermal like lining of the cyst wall and pleomorphic malignant lesions in the underlying stroma (H-E x 40).
Figure 3: Medium power view showing the highly atypical tumor cells and the presence of atypical mitosis (H-E x100).
Figure 4: High power view of the rhabdomyoblasts (H-E x200).
Figure 5: Immunohistochemical stain for desmin, showing a diffuse intense positivity of the tumor cells; this result confirms the muscular nature of the tumor (immunohistochemistry for desmin x 100).
The final diagnosis was pleomorphic rhabdomyosarcoma in the wall of a mature dermoid cyst (teratoma).
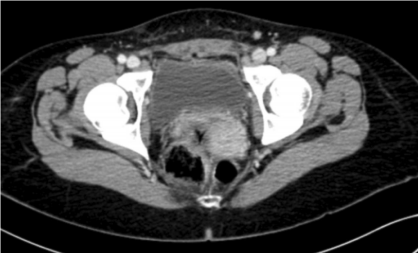
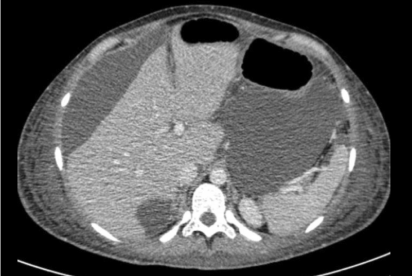
After adnexectomy, a CT scan of the abdomen did not produce any important findings (Figure 6). Based on the histological findings, a second surgery was carried out 1 month later without complications and reaching optimal cytoreduction. The patient underwent retroperitoneal para-aortic lymphadenectomy, peritoneal washings, hysterectomy, right adnexectomy, supracolic omentectomy, and pelvic lymphadenectomy by laparoscopy. Suspicious para-aortic lymph nodes and at least 4 peritoneal masses suggesting metastasis were found and removed (pouch of Douglas, abdominal wall, and cecum). A histopathologic study discovered macroscopic omental and peritoneal metastasis of pleomorphic rhabdomyosarcoma and no malignancy in the uterus, right adnexa, peritoneal washings, or any of the 19 para-aortic and 24 pelvic lymph nodes removed.
Figure 6: CT Scan: Pelvis with no tumor masses after cytoreduction.
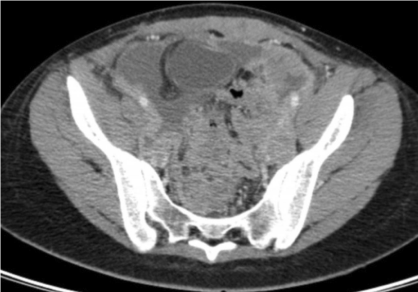
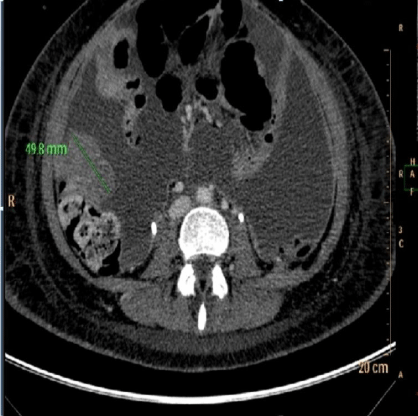
Postoperatively, the patient received a first cycle of adjuvant chemotherapy using vincristine 1,5mg/m², actinomycin D 0,45mg/ kg, and cyclophosphamide 1200 mg/m² 1 month after surgical cytoreduction. CT images obtained at the time showed several pelvic and peritoneal masses (the largest was located in the pelvis and measured 10 cm) in contact with the rectum and transverse colon. Additional findings were moderate ascites and metastasis of the anterior abdominal wall muscles (Figure 7a and 7b).
Figure 7a: CT Scan: Pelvic mass 1 month after surgery.
Figure 7b: CT scan: Ascites 1 month after surgery.
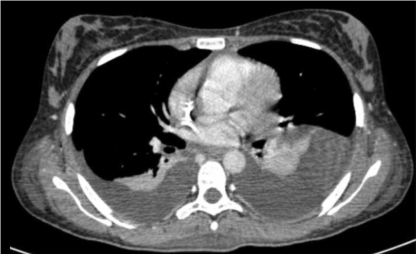
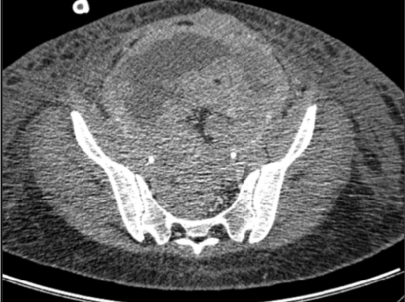
She underwent hospital admission a few days later due to bowel obstruction and fever. During the time of hospitalization she developed renal failure, for which she was treated with bilateral percutaneous nephrostomies. She received another chemotherapy cycle and yet she had disease progression. Less than 1 month later, a CT scan revealed massive pleural effusion, ascites, bilateral hydronephrosis, and several tumor masses in the peritoneum, pelvis, abdominal wall, and vaginal cuff (Figures 8a, 8b and 8c). The patient died in January 2012.
Figure 8a: CT scan: Massive pleural effusion after first cycle of chemotherapy.
Figure 8b: CT scan: Pelvis mass after first cycle of chemotherapy.
Figure 8c: CT scan: Abdominal wall metastases after first cycle of chemotherapy.
Discussion
Mature teratoma is the most common ovarian germ tumor in patients between the ages of 20 and 30 years, representing 20% of ovarian neoplasms. They are usually benign and very prevalent, so surgery is often delayed, especially in young women with small tumors [1].
Malignant transformation occurs in 1%-2% of mature cystic teratomas with a poor prognosis even with aggressive treatment. The mechanism seems to be related to the long-term presence of non-resected tissue of mature cystic teratoma [2]. In this case, the most possible explanation is that there were malignant transformations of mature cystic teratoma in the primary tumor, according to histopathological findings. Most cases that undergo malignant transformation are squamous-cell carcinomas (75-80%), though many other histological types have been described (adenocarcinoma, adenosarcoma, carcinoid…). Tumor type other than squamous-cell carcinomas is considered an indicator of worse prognosis. Early diagnosis of malignant transformation is very difficult even intraoperatively. Adjuvant therapy has not yet been standardized [3].
Prognosis of malignant teratoma is poor when it is spread beyond the ovary, and most cases are diagnosed in stages III or IV [4]. Diagnosis of malignant transformation is very difficult even intraoperatively. Risk factors for malignancy are increased patient age, tumor size, rapid growth, certain imaging characteristics, and tumor markers. According to Mori et al. [5] malignant transformation occurs an average of 10 years after the development of mature cystic teratoma. Patients over 40 years of age are at a high risk of malignant transformation. Tumor size over 10 cm is a risk factor for malignancy. In relation to the imaging examinations, the presence of solid component, obtuse angle between soft component and the inner wall, enhancement of the wall, irregular inner border, ascites, and extension through the teratoma wall as seen in MRI can be useful in the preoperative diagnosis of malignant transformation [6,7]. Neovascularization and low-resistance blood flow on solid component in color Doppler ultrasound may also be cause for suspicion [4]. Malignant tumors are often associated with hemorrhage and necrosis. The use of tumor markers is controversial and depends on histological findings. SCC seems to be the most useful marker for squamous cell components [8]. Ca 19.9, Ca 125, AFP, and CEA have also been studied with variable results. Yosioka [9] identified P 53 overexpression in 4 cases of squamous malignant transformation but not in benign cystic teratomas.
In this case, although tumor size was over 10 cm, there were no other signs of malignancy, so it was initially treated as a benign solid/ cystic teratoma and therefore the surgery performed on the patient was careful but incomplete for the final diagnosis. As there was no evidence of abdominal or pelvic disease during surgery and by TC findings, it is possible that microscopical disease was left.
As malignant transformation of mature cystic teratoma is infrequent, there are no treatment guidelines. Complete tumor excision and adequate staging can improve survival and must be performed the first surgery or as soon as possible after diagnosis. As complete laparoscopic surgery is difficult without shedding, a laparotomy is usually recommended if malignancy is confirmed or strongly suspected [4]. Adjuvant therapy has not been yet standardized. Tumors must be treated according to histological differentiation by employing a combination of surgery and chemotherapy. Even with optimal cytoreduction and adjuvant therapy, prognosis is usually poor [4]. The most important prognostic factors are the stage of the disease, presence of capsular rupture, tumor grade, vascular invasion, growth pattern, and histological type different from squamous carcinoma [1].
Our patient underwent a left adnexectomy by laparotomy because malignant transformation was not suspected. Despite complete tumor excision and staging was performed by laparoscopy, surgery was apparently optimal. Stage, high tumor grade, and histological type were very poor prognostic factors in this case. Although adjuvant therapy is not standardized, VAC (vincristine, actinomycin D, cyclophosphamide) is a good choice for immature teratoma treatment [10,11]. Radiotherapy is often used in cases of incomplete surgical resection [12]. Whether or not radiotherapy can improve survival is controversial, except in case of initial embryonal rhabdomyosarcoma, in which it is undisputed that there is no therapeutic advantage [13].
We report the case of a stage IIIb rhabdomyosarcoma (FIGO 2009) arising from a cystic teratoma with abdominal metastasis. Ovarian rhabdomyosarcoma is a rare tumor both primary and from malignant transformation of a mature cystic teratoma. Prognosis is similar and much worse than genital rhabdomyosarcomas outside the ovary [14]. A few cases of rhabdomiosarcoma arising in a mature cystic teratoma have been reported previously, some of which were diagnosed at the moment of recurrence and all having very poor prognosis [15]. The most recent case was published by Kefeli et al With only 3 months follow-up [16].
References
- Park JY, Kim DY, Kim JH, Kim YM, Kim YT, Nam JH, et al. Malignant transformation of mature cystic teratoma of the ovary: experience at a single institution. Eur J Obstet Gynecol Reprod Biol. 2008; 141: 173-178.
- Rim SY, Kim SM, Choi HS. Malignant transformation of ovarian mature cystic teratoma. Int J Gynecol Cancer. 2006; 16: 140-144.
- Sakuma M, Otsuki T, Yoshinaga K, Utsunomiya H, Nagase S, Takano T, et al. Malignant transformation arising from mature cystic teratoma of the ovary: a retrospective study of 20 cases. Int J Gynecol Cancer. 2010; 20: 766-771.
- Dos Santos L, Mok E, Iasonos A, Park K, Soslow RA, Aghajanian C, et al. Squamous cell carcinoma arising in mature cystic teratoma of the ovary: a case series and review of the literature. Gynecol Oncol. 2007; 105: 321-324.
- Mori Y, Nishii H, Takabe K, Shinozaki H, Matsumoto N, Suzuki K, et al. Preoperative diagnosis of malignant transformation arising from mature cystic teratoma of the ovary. Gynecol Oncol. 2003; 90: 338-341.
- Park SB, Kim JK, Kim KR, Cho KS. Preoperative diagnosis of mature cystic teratoma with malignant transformation: analysis of imaging findings and clinical and laboratory data. Arch Gynecol Obstet. 2007; 275: 25-31.
- Yamaoka T, Togashi K, Koyama T, Fujiwara T, Higuchi T, Iwasa Y, et al. Immature teratoma of the ovary: correlation of MR imaging and pathologic findings. Eur Radiol. 2003; 13: 313-319.
- Futagami M, Yokoyama Y, Mizukami H, Shigeto T, Mizunuma H. Can malignant transformation in mature cystic teratoma be preoperatively predicted? Eur J Gynaecol Oncol. 2012; 33: 662-665.
- Yoshioka T, Tanaka T. Immunohistochemical and molecular studies on malignant transformation in mature cystic teratoma of the ovary. J Obstet Gynaecol Res. 1998; 24: 83-90.
- Crist WM, Anderson JR, Meza JL, Fryer C, Raney RB, Ruymann FB, et al. Intergroup rhabdomyosarcoma study-IV: results for patients with nonmetastatic disease. J Clin Oncol. 2001; 19: 3091-3102.
- Kawai M, Kano T, Furuhashi Y, Iwata M, Nakashima N, Imai N, et al. Immature teratoma of the ovary. Gynecol Oncol. 1991; 40: 133-137.
- Dagher R, Helman L. Rhabdomyosarcoma: an overview. Oncologist. 1999; 4: 34-44.
- Wolden SL, Anderson JR, Crist WM, Breneman JC, Wharam MD Jr, Wiener ES, et al. Indications for radiotherapy and chemotherapy after complete resection in rhabdomyosarcoma: A report from the Intergroup Rhabdomyosarcoma Studies I to III. J Clin Oncol. 1999; 17: 3468-3475.
- Allende DS, Yang B. Primary ovarian rhabdomyosarcoma with heterologous elements: a case report. Int J Gynecol Pathol. 2008; 27: 402-406.
- Yanai H, Matsuura H, Kawasaki M, Takada Y, Tabuchi Y, Yoshino T. Immature teratoma of the ovary with a minor rhabdomyosarcomatous component and fatal rhabdomyosarcomatous metastases: the first case in a child. Int J Gynecol Pathol. 2002; 21: 82-85.
- Kefeli M, Kandemir B, Akpolat I, Yildirim A, Kokcu A. Rhabdomyosarcoma arising in a mature cystic teratoma with contralateral serous carcinoma: case report and review of the literature. Int J Gynecol Pathol. 2009; 28: 372-375.