Case Report
Austin J Clin Case Rep. 2014;1(11): 1051.
Fulminant Evolution of Stomach Cancer during Pregnancy
González-Mesa E*, Armenteros MA, Molina A and Herrera J
Malaga University Hospital, Malaga University School of Medicine, Spain
*Corresponding author: Ernesto González-Mesa, Malaga University Hospital, Arroyo de los Angeles St, 29011-Málaga, Spain
Received: September 10, 2014; Accepted: September 22, 2014; Published: September 24, 2014
Abstract
The incidence of stomach cancer associated to pregnancy has been reported as high as 1/1000, especially in some Asiatic regions. Its diagnosis poses a challenge for obstetricians since even in the advanced stages of the disease it is clinically characterized by nonspecific gastrointestinal symptoms such as epigastric pain, nausea, vomiting or early satiation, among other manifestations. Such alterations are easily attributable to the discomfort normally associated to pregnancy. We present the case of a 33-years old Caucasian pregnant woman, with an uneventful pregnancy until the moment that a gastric cancer was diagnosed in the 26th week of her fifth pregnant. A fulminant and catastrophic evolution followed the diagnosis.
Keywords: Gastric cancer; Cancer and pregnancy; Maternal death; High risk pregnancy
Abbreviations
CT: Computed Tomography; MRI: Magnetic Resonance Imaging; PCI: Peritoneal Carcinomatosis Index
Introduction
The incidence of stomach cancer associated to pregnancy is estimated to be 1/1000, though the existing data are inconclusive. In the last three decades there has been a notorious increase in the number of stomach cancers diagnosed in pregnant women – one of the underlying causes being the increase in maternal age during this period of time. Another possible cause is the fact that pregnancy is now subjected to closer monitoring than in the past, and this facilitates the diagnosis of cases that were previously not detected [1].
To date it has not been possible to demonstrate that pregnancy acts as a cause or risk factor for the development of cancer. In fact, the incidence of malignant disease in pregnant women is similar to that observed in non-pregnant women of the same age group [1]. Haas [2] demonstrated a lower incidence than expected of all cancers in pregnant women and speculated that women with subclinical cancers do not usually become pregnant, presumably due to decreased libido resulting from constitutional symptoms. It has also been suggested that conception, implantation, or early embryonic development could be disrupted by hormonal or immunological factors concomitant to malignant disease.
The diagnosis of cancer during pregnancy poses a challenge from the medical, personal, social and moral perspectives. In effect, we have a conflictive situation: on one hand we need to treat the malignancy in order to improve the maternal prognosis, and on the other hand we need to continue pregnancy in order to improve the fetal prognosis. The management of these two patients therefore requires a multidisciplinary approach involving obstetricians, oncologists and psychologists, among others.
The most frequent tumor locations in pregnant women are the skin, cervix, breast, hematological system, ovary and colon. In this respect, stomach cancer represents 0.1% of the cancers diagnosed in pregnant women [1].
Case Report
A 33-year-old Caucasian pregnant woman presented to the Emergency Room of Málaga University Hospital in week 25+1 of her fifth pregnancy, feeling some contractions and abdominal discomfort. This was her fifth pregnancy. She had three prior miscarriages (negative thrombophilia study), and one spontanous 28 weeks preterm delivery due to preterm premature rupture of membranes, three years before.
She belonged to a family of Romanian in migrants that came to Spain six months ago. In her family history, her father was suffering from lung tuberculosis and cirrhosis. Her mother committed suicide some years before. She smoked for years near a pack of cigarettes a day, although she reduced during pregnancy up to 7 cigarettes a day.
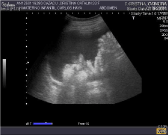
The patient was admitted for clinical observation due to a funneled cervix measuring 17-19 mm and irregular contractions, with no other symptoms. The blood tests revealed anemia (hemoglobin 10.4 g/dl) and a C-reactive protein concentration of 108 mg/L as isolated findings. On the second day of admission the patient developed abdominal discomfort, fullness sensation and bloating. Abdominal ultrasound and complete blood tests were requested due to progression of the abdominal bloating and worsening of the clinical condition. The parameters were again found to be within normal ranges, with normal transaminase and blood amylase levels, except for a C-reactive protein increase to 137 mg/L and a platelet count of 585,000/mm3. While in wait of the abdominal ultrasound report, obstetric ultrasound was performed, revealing a fetus in the cephalic position with correct biometric data, an estimated fetal weight of 900 g, and normal placental and amniotic fluid data. The cervical length was 13 mm, and ascitic fluid in moderate amounts was observed in both flank regions (Figure1). Abdominal ultrasound revealed apparent thickening of the gastric wall and pancreas (more pronounced in the tail region), with no evidence of any solid mass. Important anechoic ascitic fluid was noted, with no presence of septae, located predominantly in the perihepatic and perisplenic regions, in both flanks, and in the upper retroperitoneum.
Figure 1: Abdominal ultrasound showing important ascites.
Diagnostic paracentesis was decided to evaluate the existence of infectious, inflammatory or neoplastic disease. Leukocytes without germs were identified. Prophylactic intravenous antibiotic treatment was prescribed (cefuroxime 1.5 g/8 hours), in wait of the definitive results.
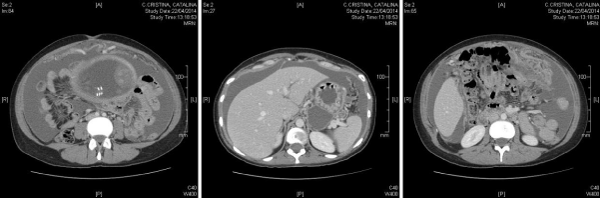
MRI and CT (Figure 2) were requested due to the suspicion of a possible gastric tumor, revealing a probable gastric neoplasm with peritoneal carcinomatosis and severe ascites. A biopsy was performed under endoscopic guidance. A multidisciplinary committee comprising the Departments of Gynecology, General Surgery, Oncology, Radiology and Radiotherapy decided palliative chemotherapy, since curative surgical management was discarded. The maintenance of pregnancy was proposed with a view to securing a greater gestational age, with evacuating paracentesis to afford symptoms relief.
Figure 2: Left: omental mass. Center: tumor mass at gastric level. Right: important ascites.
The patient suffered important daily worsening despite the supportive measures, with deterioration of the gastrointestinal manifestations and oliguria. Parenteral nutrition was started and a permanent abdominal catheter was placed for draining the ascitic fluid.
On the eighth day of admission we received the pathologist report, which confirmed the suspected diagnosis: diffuse, poorly differentiated signet ring cell adenocarcinoma.
Palliative chemotherapy was scheduled. However, the patient condition continued to worsen, and although the fetus had shown reactive cardiotocographic recordings until then, we now began to record scant activity. Cesarean section was therefore decided. It was performed at 26+3 weeks, and a male newborn was delivered with 800 g and good Apgar scores, being admitted in neonatal intensive care unit.
In the operation we found ascites (1400 ml), peritoneal carcinomatosis with a Peritoneal Carcinomatosis Index (PCI) of 23. We also found an omental mass at greater epiploon level, a gastric neoplasm infiltrating the pancreas, and tumor implants in uterine surface and left Fallopian tube. Biopsies were collected and sent to the Department of Pathology, along with the placenta.
Following surgery, the patient was admitted to the intensive care unit for monitoring and recovery, with a view to administering chemotherapy as soon as possible. However, the patient condition had worsened greatly, and death occurred on fifth day of puerperium. The newborn was discharged from the hospital eight weeks later and at the moment suffers retinal sequelae in one eye due to prematurity.
Discussion
The incidence of stomach cancer during pregnancy is very low. According to the literature, which is concentrated particularly in Japan, the estimated incidence is 0.016% of all pregnancies [3]. It is important to mention that although most published cases correspond to Japanese patients, the data from the Japanese studies should be extended to the rest of the world population.
Early-stage stomach cancer is generally asymptomatic. Advanced stages of the disease are typically characterized by nonspecific gastrointestinal symptoms such as epigastric pain, nausea, vomiting or early satiation, among other manifestations. Such alterations are easily attributable to the discomfort normally associated to pregnancy. Other frequent forms of presentation comprise anemia, diminished weight gain or even weight loss. Although infrequent, the malignancy can also manifest in complicated form such as for example peritonitis secondary to gastric perforation. Therefore, in the case of a pregnant woman with persistent gastrointestinal discomfort, particularly in the presence of digestive bleeding, weight loss or persistent vomiting beyond week 20 of pregnancy, the differential diagnosis should include stomach cancer, with the indication of prompt endoscopic evaluation [4,5]. Biopsies for histological study are required if suspect lesions are identified.
Regarding the role of endoscopy and its safety in pregnancy, there is general agreement that its benefits in evaluating gastrointestinal alarm symptoms in pregnant patients outweigh the potential risks (generally derived from the sedating drugs used to perform the technique). Endoscopy is thus justified in the abovementioned cases [6].
In the same way as the rest of stomach cancers detected in young individuals, the tumors found in these patients are histologically characterized as poorly differentiated adenocarcinomas – a fact that worsens the prognosis [1].
At the time of the diagnosis, the great majority of pregnant patients present advanced stomach cancer. This is partly because of the delay in establishing the diagnosis, since the symptoms tend to be attributed to the gestational process [7]. In the review published in 2009 by Sakamoto et al. [4], involving the evaluation of 137 cases of stomach cancer in pregnant Japanese women, 92.5% had advanced stage disease at the time of the diagnosis [4]. Similar results have been reported in the review published by Jaspers et al. referred to the general population, in which 100% of the stomach malignancies were found to be in advanced stages [8].
The management of stomach cancer in pregnant patients is fundamentally dependent upon the gestational age and tumor stage [9]. Other aspects to be taken into account are the evolution of pregnancy and the maternal health condition.
Ueo et al. [9] proposed in 1991 a diagnostic algorithm that has been accepted by most of the current clinical guides [7]. Patients amenable to surgery are divided into four groups according to gestational age:
Group 1: Gestational age under 24 weeks. Surgery is indicated regardless of the condition of pregnancy.
Group 2: Gestational age between 25 and 29 weeks. Surgery is indicated in the event of advanced-stage but resectable disease. However, in early-stage tumors, surgery can be postponed until week 30 to ensure fetal viability and improve the perinatal outcome.
Group 3: Gestational age over 30 weeks. Pregnancy should be ended via the vaginal route or cesarean section, followed by surgery.
Group 4: Postpartum. Management similar to that indicated in the general population.
In the event of complications (bleeding, perforation, peritonitis, etc.), surgery should be performed regardless of the condition of pregnancy or the gestational age.
The administration of chemotherapy (whether adjuvant or palliative) is possible after the first three months of pregnancy. In this regard, there are no data suggesting an increased risk of fetal loss or anomalies, though an increased incidence of prematureness and intrauterine growth retardation has been described [7]. The main chemotherapeutic agents are 5-FU, anthracyclines and platinum drugs [1,7].
The prognosis in these patients is poor, due to the advanced stage of the disease at the time of diagnosis. In general, the survival rate after one year is about 20%, versus 15% after two years [4]. In advanced disease the figures are even poorer.
The estimated fetal survival rate is 70% and improves with advancing gestational age – with rates of close to 100% in fetuses beyond a gestational age of 30 weeks. On the other hand, although the placenta can be affected by metastatic disease [10], there is no evidence to suggest that tumor spread can directly affect the fetus [7].
References
- Pentheroudakis G, Pavlidis N. Cancer and pregnancy: poena magna, not anymore. Eur J Cancer. 2006; 42: 126-140.
- Haas JF. Pregnancy in association with a newly diagnosed cancer: a population-based epidemiologic assessment. Int J Cancer. 1984; 34: 229-235.
- Yoshida M, Matsuda H, Furuya K. Successful treatment of gastric cancer in pregnancy. Taiwan J Obstet Gynecol. 2009.
- Sakamoto K, Kanda T, Ohashi M, Kurabayashi T, Serikawa T, Matsunaga M, et al. Management of patients with pregnancy-associated gastric cancer in Japan: a mini-review. Int J Clin Oncol. 2009; 14: 392-396.
- Hagen A, Becker C, Runkel S, Weitzel HK. Hyperemesis in late pregnancy--should we think of cancer? A case report. Eur J Obstet Gynecol Reprod Biol. 1998; 80: 273-274.
- Cappell MS. The fetal safety and clinical efficacy of gastrointestinal endoscopy during pregnancy. Gastroenterol Clin North Am. 2003; 32: 123-179.
- Dunkelberg JC, Barakat J, Deutsch J. Gastrointestinal, pancreatic, and hepatic cancer during pregnancy. Obstet Gynecol Clin North Am. 2005; 32: 641-660.
- Jaspers VK, Gillessen A, Quakernack K. Gastric cancer in pregnancy: do pregnancy, age or female sex alter the prognosis? Case reports and review. Eur J Obstet Gynecol Reprod Biol. 1999; 87: 13-22.
- Ueo H, Matsuoka H, Tamura S, Sato K, Tsunematsu Y, Kato T. Prognosis in gastric cancer associated with pregnancy. World J Surg. 1991; 15: 293-297, discussion 298.
- Baker AM, Haeri S, Shafer A, Moldenhauer JS. Maternal gastric carcinoma metastatic to the placenta. Eur J Obstet Gynecol Reprod Biol. 2010; 153: 225-226.