1Penn State Hershey Cancer Institute, USA
2Department of Pathology, Pennsylvania State University College of Medicine, USA
*Corresponding author: Jeffrey J. Pu, Department of Pathology, Penn Hershey Cancer Institute, Penn State College of Medicine, Mail Code H064, RoomC6602C, 500 University Drive, P.O. Box 850, Hershey, PA 17033-0850, USA
Received: October 30, 2014; Accepted: December 01, 2014; Published: December 03, 2014
Citation: Pu JJ, Chepovetsky J and Truica CI. A Rare Case of Synchronous Breast Carcinoma and Mantle Cell Lymphoma: Successful Treatment of Both Cancers with Bendamustine/Rituxan Combination. Austin J Clin Case Rep. 2014;1(12): 1056. ISSN : 2381-912X
We present a rare case of a 64 year old female with a synchronous diagnosis of breast carcinoma and ipsilateral, diffuse mantle cell lymphoma. At the time of diagnosis, the lymphoma was deemed to pose more of an overall risk to the patient and an initial treatment with bendamustine/Rituxan was begun. After 3 cycles, a repeat PET CT showed a significant decrease in tumor burden from the lymphoma as well as a significant reduction of the breast carcinoma tumor size and FDG avidity. A literature review showed some evidence that the pathogenesis of breast carcinoma and mantle cell lymphoma may share some common pathways. Further research into the effectiveness of Bendamustine as an adjuvant chemotherapy regiment for patients with synchronous breast carcinoma could prove useful in clinical practice.
Keywords: Breast Carcinoma; Mantle Cell Lymphoma; Bendamustine
PET-CT: Positron Emission Tomography–Computed Tomography; FDG: Fluorodeoxyglucose; BMI: Body Mass Index; ER: Estrogen Receptor; PR: Progesterone Receptor; HER2: Human Epidermal Growth Factor Receptor 2; CEP 17: Chromosome 17 Centromere
Breast carcinoma is the most common malignancy in women (excluding skin cancer) and its incidence increases with age such that majority of breast carcinomas develop in women older than 55 years. With improvements in screening and treatment, the mortality from breast carcinoma has decreased and more women are surviving their initial breast carcinoma diagnosis. A personal history of breast carcinoma increases the risk of subsequent breast carcinoma diagnosis, and many women who survive their initial diagnosis present with second breast carcinoma primaries in same breast or contra-lateral breast. The increased incidence of cancer with age, and the routine use in staging of high sensitivity CT scanning and/or PET scanning has made the synchronous finding of two or more cancers increasingly common, confronting physicians and patients with new challenges in treatment decisions. Staging of two different synchronous cancers can be a challenge especially when the lymph node drainage of the two malignancies overlaps. Synchronous diagnosis of breast carcinoma with carcinomas of the thyroid, lung and colon are becoming increasingly common. The initial staging and determining the prognosis of both cancers is important in determining the treatment plan for best overall outcome. Often physicians elect to address the most aggressive malignancy first.
Here we present the case of a patient who had survived a diagnosis of breast carcinoma 14 years prior and presented with a new synchronous diagnosis of contra-lateral breast carcinoma and systemic mantle cell lymphoma.
A 64 year old female presented to her family doctor with 15-pound weight loss in 6 months, generalized body aches, fatigue and dyspnea on exertion. She had a 40 years history of smoking, and a history or right sided breast carcinoma treated with mastectomy and radiation approximately 14 years prior. Anastrozole had been prescribed for 5 years for her breast carcinoma but she had only taken it for about 3 years. Her only medications where ibuprofen as needed for chronic back pain. On exam she was frail, afebrile, her weight was 102 pounds, BMI-20. Lung examination showed decreased air entry, wheezing and ronchi bilaterally. She had diffuse palpable lymphadenopathy in the neck, bilateral axillary areas, epitrochlear areas, sides of her flank, and bilateral inguinal regions. A left breast 2 cm mass was palpable in the 10 to 12 o’clock position. The left nipple was retracted but the skin had no erythema or peaud’orange changes. Her right mastectomy scar was well-healed without palpable evidence of local recurrence. Abdominal exam revealed splenomegaly 3cm below the costal margin and lower extremities had 1+ pitting edema.
She underwent a diagnostic mammogram that showed a high density irregular mass with ill-defined margins and innumerable pleomorphic calcifications in the left breast upper inner quadrant 11 o’clock middle depth and a second high density irregular mass at 10 o’clock posterior depth. There were multiple high density oval masses in the left axilla. Ultrasound examination showed an irregular mass with anti-parallel orientation measuring 15 mm in left breast upper inner quadrant 11 o’clock middle depth and a second mass measuring 12 mm at 10 o’clock posterior depth as well as multiple enlarged lymph nodes with thickened cortices in left axilla. The right axilla and retropectoral area also had abnormal lymph nodes.
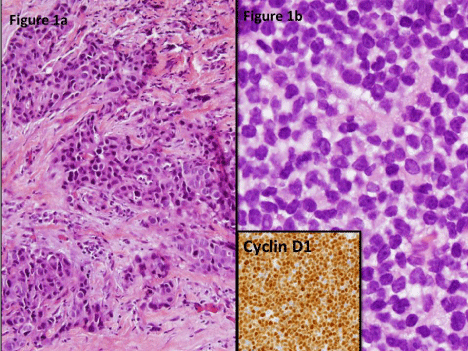
A biopsy of the left breast 11 o’clock mass showed invasive ductal carcinoma in 4 out of 4 cores, measuring 1.1 cm, grade II, with associated carcinoma in situ. The tumor was ER and PR negative. HER 2 was assayed via interphase in situ hybridization (INFORM Dual ISH) and was positive with a Her 2: CEP 17 signal ratio of 2.09 and average Her 2 DUAL ISH signals per nucleus of 4.44 (Figure1).
She underwent chest and abdominal CT scans to assess for tumor burden which revealed extensive and diffuse lymphadenopathy above and below the diaphragm as well as a 7 mm left lower lobe lung mass, all concerning for metastatic disease. In addition, there was moderate splenomegaly, potentially indicating metastatic disease.
A left axillary lymph node biopsy and an inguinal lymph node biopsy both showed an atypical monomorphic small lymphocyte population suspicious for lymphoma. Immunostaining showed that the neoplastic cells stained positively for CD20, PAX-5, Cyclin D1 and bcl2; and negatively for bcl6. Ki67 highlighted 2-30% of neoplastic nuclei. Flow cytometry showed a CD5+, kappa-light chain restricted monotypic B cell population. The B lymphocytes also co-expressed CD22 and CD38, and were negative for CD10 and CD23; consistent with a diagnosis of mantle cell lymphoma.
Because the patients symptoms were quite severe and related to the lymphoma (fatigue, shortness of breath, early satiety from splenomegaly) while her breast carcinoma was localized to the breast, she was initially started on benadmustine (120mg/m2 IV on day 1 and 2 of a 21-day cycls) and rituxan (375mg/m2 IV on day 1 of a 21-day cycles) combination therapy.
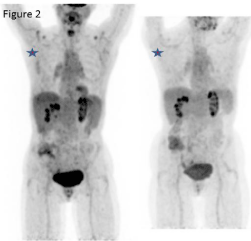
After 3 cycles of chemotherapy a repeat PET/CT scan showed significant decrease in the diffuse lymphadenopathy as well as decreased spleen size from 17.6 cm to 11.5 cm. The left breast soft tissue mass was also significantly smaller and was minimally FDG avid (Figure 2). Symptomatically the patient felt better with improved energy, appetite and weight. She completed 4 cycles of bendamustine and rituxan and subsequently started maintenance rituxan treatment. Approximately 5 months from her diagnosis she underwent a left total mastectomy. A single focus of residual invasive ductal carcinoma, high grade, was found, measuring 2mm. Resection margins were negative and eight lymph nodes were negative for metastatic carcinoma (final TNM stage ypT1a, ypN0). Post-operatively she began adjuvant chemotherapy with weekly paclitaxel and trastuzumab for 12 weeks followed by trastuzumab every three weeks. She tolerated the chemotherapy well and continued to improve symptomatically 7 months after left total mastectomy and 8 months after chemotherapy for systemic mantle cell lymphoma.
We present a rare case of synchronous diagnosis and treatment of early stage breast carcinoma and mantle cell lymphoma. As per literature review, this is the third case report of a patient with breast carcinoma and mantle cell lymphoma; and the first case of synchronous diagnosis of new breast carcinoma and systemic mantle cell lymphoma [1,2].
Hematologic malignancies such as Acute Myelogenous Leukemia (AML) and Myelodysplastic Syndromes (MDS) after breast carcinoma chemotherapy and radiation therapy have been abundantly reported. However, treatment-related mature B-cell neoplasms are rare. Mantle cell lymphoma is a subtype of small B cell lymphoma with a unique immunohistochemical and cytogenetic phenotype. It is an aggressive neoplasm and the median overall survival rate for this lymphoma is 3~5 years [3]. Her 2 + breast carcinoma is the most aggressive type of breast carcinoma, though novel Her 2 based therapies have significantly improved Stage I Her 2+ disease survival to 96~98% in the last decade. We decided to use Bendamustin/Rituxan combination regimen to treat the mantle cell lymphoma first because the patient’s symptoms were likely due to extensive lymphoma rather than her limited breast cancer.
Bendamustine is a unique cytotoxic agent with structural similarities to alkylating agents and antimetabolites, though it is non– cross-resistant with alkylating agents and other drugs in vitro and in the clinic. Rituximab (trade names Rituxan, MabThera and Zytux) is a chimeric monoclonal antibody against the protein CD20, which is primarily found on the surface of immune system B cells. Two North American trials suggested that bendamustine may be the most effective drug available for indolent Non-Hodgkin’s Lymphoma (NHL) [4,5]. The combination therapy of bendamustine and rituximab showed a response rates of 90% to 92% and complete remission of 55% to 60% in patients with follicular and mantle-cell lymphoma. Previous investigation indicated that bendamustine is effective in treating some solid tumors such as breast carcinoma as well [5]. There were 2 phase II trials using bendamustine as one of chemotherapy regimens for breast carcinoma treatments [6,7]. However, in those 2 studies only a minority (10%) of patients enrolled were Her 2 positive. Given the excellent tolerability of bendamustinein our patient and previous trials and the accumulating data on its effectiveness in breast cancer we believe that further studies with combination Her 2 targeted therapy and bendamustine in Her 2 + breast cancer are warranted.
We also performed a literature search to determine possible links between the pathogenesis of breast cancer and mantle cell lymphoma. Subramaniam et al reported that the oncogenic Epstein-Barr virus may be responsible for spread of both invasive breast carcinoma and lymphomas through the nuclear protein EBNA-3C interacting with the human metastatic suppressor protein Nm23-H1 reversing its ability to stop breast and lymphoma cell migration [8]. It was reported that CX3CR1, a non-B cell adhesion molecule, may be expressed on mantle cell lymphoma and aid in lymphoma dissemination in breast carcinoma patients [9]. A study also showed that inactivation of a single gene within the BRCA pathway can increase the risk of developing mantle cell lymphoma [10]. Based upon above-mentioned evidence, the synchronous occurrence of both breast carcinoma and mantle cell lymphoma may beyond simple secondary cancer theory and actually related to a common etiology. Research needs to be done to further explore the correlative pathogenesis between those two cancers.
Our experience of creating a treatment algorithm for these synchronous malignancies may help oncologists to make therapeutic strategies in treating other synchronous cancers. These decisions should be made based on the patient’s symptoms, tumor burden, histologic and molecular assessment of both tumors and the side effect profile of all proposed chemotherapeutic agents. We hope that our experience in this case may provide further incentive for the possibility of studying these drugs in the treatment of solid tumors.
We would like to thank Eleanor Gerhart for help with literature searches.
Figure 1a: Left breast core biopsy showing invasive breast ductal carcinoma (H&E).
Figure 1b: Left axillary lumph node biopsy showing monomorphous lymphoid population with strong immunohistochemical expression of cyclin D1(inset).
Initial PET CT( left) scan showing diffuse axillary lymphadenopathy and FDG avid left breast soft tissue mass. Post-treatment (bendamustine/rituxan) PET CT (right) showing decreased FDG avidity in breast mass.