1Department of Internal Medicine, Division of Gastroenterology, Otsu Red Cross Hospital, Japan
2Department of Internal Medicine, Division of Nephrology, Otsu Red Cross Hospital, Japan
*Corresponding author: Tetsuya Makiishi, Department of Internal Medicine, Division of Nephrology, Otsu Red Cross Hospital, 1-1-35 Nagara, Otsu, Shiga 520-8511, Japan
Received: September 30, 2014; Accepted: December 03, 2014; Published: December 04, 2014
Citation: Yoshikawa T, Makiishi T, Yabuuchi J, Nobuta H and Maeda S. Penicillin Induced Pseudo-Hypoalbuminemia. Austin J Clin Case Rep. 2014;1(12): 1058. ISSN : 2381-912X
A 75-year-old man on regular hemodialysis was admitted to our hospital with a 3-week history of low-grade fever and mild loss of appetite. The patient was ultimately diagnosed with infective endocarditis caused by Streptococcus salvarius, and treatment with a 4-week course of intravenous penicillin G 3 MU every 6 hours and a 1-week course of intravenous gentamicin was initiated. The patient’s symptoms resolved soon after treatment began; however, his serum albumin concentration, measured using a modified Bromocresol Purple (BCP) assay, fell progressively to 0.9 g/dL after 3 weeks of therapy, although serum total protein concentration remained stable. We re-measured the serum albumin concentration with a Bromocresol Green (BCG) assay, and also calculated it from the fraction of albumin in the total amount of protein assessed by electrophoresis, which yielded results of 2.7 g/dl and 2.9 g/dL, respectively. We concluded that the extremely low albumin concentration was an artifact caused by a measurement error of the modified BCP assay. The modified BCP assay is becoming an increasingly popular means of measuring albumin concentration, but there are reports that it underestimates albumin concentration in the presence of high concentrations of penicillin G. When evaluating a patient with hypoalbuminemia who is treated with penicillin G, clinicians should take into account the assay used at their institution, and, if the modified BCP assay is used, should confirm the result with other assays.
Keywords: Penicillin G; Hypoalbuminemia; Bromocresol purple
Hypoalbuminemia is a common finding in individuals with acute and chronic medical conditions, affecting 20% of patients admitted to hospital [1]. Hypoalbuminemia can be caused by a wide variety of conditions, including nephrotic syndrome, hepatic cirrhosis, heart failure, malnutrition, and acute and chronic inflammatory diseases [2]. However, there have been few reports of hypoalbuminemia resulting from measurement error, or pseudo-hypoalbuminemia. In this report, we describe a case of pseudo-hypoalbuminemia in a patient dependent on hemodialysis, who developed infective endocarditis and was treated with penicillin G. We also discuss the mechanism of pseudo-hypoalbuminemia and its clinical significance.
A 75-year-old Japanese man was admitted to our hospital with a 3-week history of low-grade fever and mild loss of appetite. He had been on maintenance hemodialysis (three sessions per week) for 10 years, as a consequence of end-stage kidney disease secondary to hypertensive nephrosclerosis. He had been treated as an outpatient for hypertension for 20 years and for gastroesophageal reflux disease for 2 years at the dialysis center affiliated to our hospital. He had undergone aortic valve replacement with xenograft for severe aortic stenosis 10 months before admission. At the time of admission he was taking olmesartan (20 mg/day), amlodipine (5 mg/day), omeprazole (10 mg/day), warfarin (2 mg/day) and calcium carbonate (1,500 mg/ day). On admission, his blood pressure was 122/66 mmHg, pulse rate 77 /min, and temperature 37.4°C. Physical examination was significant only for mild conjunctival pallor. Roth’s spots, Janeway lesions or Osler’s nodes were not evident.
Laboratory investigations revealed the following: white blood cell count, 5.5 × 103/μL; red blood cell count, 2.13 × 106/μL, hemoglobin, 7.0 g/dL; hematocrit, 20.8%; platelet count, 124 × 103/μL; total bilirubin, 0.43 mg/dL; aspartate aminotransferase, 14 U/L; alanine aminotransferase, 8 U/L; lactate dehydrogenase, 204 U/L; serum creatinine, 7.16 mg/dL; blood urea nitrogen, 32.2 mg/ dL; sodium, 140 mEq/L; potassium, 3.6 mEq/L, chloride, 99 mEq/L, calcium, 7.7 mg/dL, phosphate, 3.0 mg/dL; serum albumin, 2.4 g/dL; C-reactive protein, 1.2 mg/dL; immunoglobulin (Ig) G, 1,352 mg/dL; IgA, 214 mg/dL; IgM, 133 mg/dL.
Four sets of blood cultures were collected, two on the day before admission and two on the day of admission. All were positive for Streptococcus salvarius. Chest radiography and computed tomography of the abdomen showed no abnormalities. Transesophageal echocardiography did not reveal any evidence of vegetation on any of the cardiac valves. However, diffusion-weighted magnetic resonance imaging of the brain revealed the presence of single high-intensity spot in the occipital cortex, which was consistent with a diagnosis of a septic embolus. The patient therefore fulfilled one major and three minor Duke’s criteria and was diagnosed with infective endocarditis caused by Streptococcus salvarius [3].
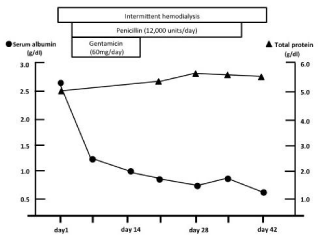
According to the guidelines for the treatment of infective endocarditis, intravenous penicillin G therapy 3 MU every 6 hours was started and maintained for 4 weeks, along with intravenous gentamicin therapy at a dose of 60 mg/day for the first 7 days [4]. On the second day of treatment, the blood culture was negative for pathogens. The patient’s low-grade fever resolved and his appetite returned within a week of beginning treatment. However, his serum albumin began to fall soon after the initiation of therapy (Figure 1).
We sought possible explanations for his persistent and worsening hypoalbuminemia. The differential diagnosis included hepatic cirrhosis, protein-losing enteropathy, nephrotic syndrome, malnutrition and a chronic inflammatory response. Among these, however, hepatic cirrhosis, protein-losing enteropathy, nephrotic syndrome and malnutrition were unlikely based on the patient’s clinical presentation, laboratory results, imaging findings and clinical course. Although a chronic inflammatory response could be caused by subacute infectious endocarditis, and would therefore explain his hypoalbuminemia, the serum albumin concentration did not show any improvement despite the patient’s positive response to antibiotics. Furthermore, our patient did not display any of the clinical signs that would be expected in an individual with such low serum albumin concentration, such as peripheral edema, pleural effusions or ascites. In addition, the serum total protein concentration remained within its normal range despite the severity of the hypoalbuminemia (Figure 1). Hence, we suspected a measurement error as a possible cause of the apparent hypoalbuminemia.
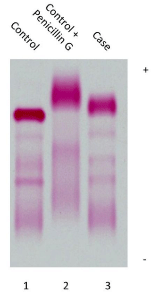
In our institution, serum albumin concentration had been measured with a modified Bromocresol Purple (BCP) assay since 2013. Before this the Bromocresol Green (BCG) assay had been used. We measured the patient’s serum albumin concentration in a sample obtained on the 23rd day of admission with both assays, which revealed that the albumin concentration measured with a modified BCP assay was 0.9 g/dL, compared with 2.7 g/dL when measured with the BCG assay. We further assessed his serum albumin concentration by electrophoresis of the same sample, and compared it with a control sample from healthy volunteer incubated in vitro with and without penicillin G. We found that the albumin band in the specimens from the patient and the healthy volunteer incubated with penicillin G were broad, and had shifted toward the cathode compared with the specimen from the healthy volunteer that had not been incubated with penicillin G (Figure 2). The patient’s serum albumin concentration calculated from the total protein concentration of 5.5 g/dL and albumin fraction of 53.2 % assessed by dye-based quantification was 2.9 g/dL, which was double that measured with the modified BCP assay, and corresponded closely to that measured with the BCG assay. Therefore, we were able to conclude that the patient had pseudo-hypoalbuminemia resulting from a measurement error in the modified BCP assay, likely influenced by treatment with high-dose penicillin G.
The patient’s recovery was otherwise uneventful, and he was discharged 48 days after admission, with a serum albumin concentration of 2.7 g/dL measured using the BCG assay.
Serum albumin concentration is typically measured with the BCG or BCP assay in Western countries [5]. In both assays, albumin concentration is determined by measuring the shift in the absorption spectrum of BCG or BCP that occurs when they bind albumin. Both assays have some limitations: the BCG assay tends to overestimate albumin concentration as a result of interference caused by the binding of serum globulin to albumin, and the BCP assay is strongly influenced by redox states of the albumin molecule [5,6]. Recently, to overcome these limitations, a modified version of the BCP assay has been developed and has been gradually replacing the traditional BCP and BCG assays [7]. However, it has recently been reported that the modified BCP assay underestimates albumin concentration in the presence of high concentration of penicillin [8].
Ono et al. reported two cases of pseudo-hypoalbuminemia in 2009, in which serum albumin concentration was measured with the modified BCP assay in two patients with infective endocarditis treated with 8–24 MU per day of penicillin G [8]. They found that in both cases the albumin concentration measured by the modified BCP assay was approximately half that expected, calculated on each patient’s total protein concentration and albumin fraction assessed by electrophoresis and dye-based quantification. They also reported that the modified BCP assay underestimated albumin concentration in a penicillin G dose-dependent and an incubation time-dependent manner in vitro. Since this first report, however, there have been few others concerning this problem. We believe that the scarcity of similar reports does not imply that the problem is not clinically significant.
A potential explanation for the relative lack of reporting of this phenomenon is that it is specific to the BCP assay, and is not an issue for the BCG assay. Nevertheless, as the modified BCP assay gains popularity and is used more widely in clinical practice, more cases of pseudo-hypoalbuminemia will likely be seen. A second explanation is that the number of clinical scenarios in which pseudo-hypoalbuminemia could occur is relatively limited, as BCP assays are thought to underestimate albumin concentration only when patients are treated with high-dose penicillin G [8]. There have been no reports that the assay is influenced by other drugs, including antibiotics related to penicillin both in vivo and in vitro. There are also relatively few indications for high-dose penicillin G treatment, the most common and important being for infective endocarditis and necrotizing fasciitis. In patients with impaired renal function or on dialysis therapy that results in reduced clearance of penicillin G – like our patient – pseudo-hypoalbuminemia might be evident at moderate or even low doses.
The final explanation for the paucity of reports is that pseudo-hypoalbuminemia may be mistaken for clinical hypoalbuminemia, as patients who require high-dose penicillin G treatment might have poor nutritional status. When evaluating the nutritional status of a patient treated with penicillin G, the serum albumin concentration should be interpreted with caution if measured with a BCP assay, and other indicators of nutritional status such as serum cholinesterase concentration should be measured at the same time, to avoid exposing patients to unnecessary investigations or treatments.
Serum albumin concentration can be underestimated when measured with the modified BCP assay in patients treated with penicillin G. Hence, when evaluating such patients, clinicians should be aware of the assay used to measure albumin concentration at their institution. If the modified BCP assay is used, it is important to confirm any abnormal findings in albumin concentration with the BCG assay, or with an estimation based on total protein concentration and albumin fraction assessed by electrophoresis.
Changes in serum albumin and total protein concentrations after admission. TP: Total Protein.
Assessment of serum albumin by electrophoresis. Lane 1, serum from a healthy volunteer; lane 2, serum from healthy volunteer incubated with 1000 unit / mL penicillin G for 12 h at 37°C; lane 3, serum from the patient. The sera were electrophoresed and stained with Ponso S. Note that the albumin fractions in lanes 2 and 3 moved toward the cathode, forming wide protein bands. +: The Cathode; −: The Anode.