
Case Report
Austin J Clin Case Rep. 2014;1(12): 1062.
Is a Long-Term Physiotherapy and Rehabilitation Program Effective in Van Der Knaap Syndrome?
Bilge Nur Yardimci, Ozgun Kaya Kara* and Akmer Mutlu
Department of Physiotherapy and Rehabilitation, Hacettepe University, Turkey
*Corresponding author: Ozgun Kaya Kara, Department of Physiotherapy and Rehabilitation, Hacettepe University, Faculty of Health Sciences, 06100 Ankara, Turkey
Received: November 06, 2014; Accepted: December 05, 2014; Published: December 08, 2014
Abstract
This study aimed to investigate the benefit of physiotherapy treatment that implemented programme from 1 year old up to 5 years in a patient with Van der Knaap Syndrome. Physiotherapy evaluations of the child included cognitive, fine and gross motor development assessed with the Bayley Scales of Infant and Toddler Development - Third Edition (Bayley-III), gross motor function with the Gross Motor Function Measurement (GMFM), and tonus evaluation with the Modified Ashworth Scale. Physiotherapy programme was performed during 36 months, 3 days per week by physical therapist according to Neurodevelopmental Treatment approach. During the therapy programme, GMFM scores are increased 19,43% to 81,14% and the cognitive, motor and language development of patient was "extremely low" level according to Bayley-III. This case report is the first study about the long-term physiotherapy programme of child with Van der Knaap and shows significant effect of this.
Keywords: Physiotherapy; Rehabilitation; Bayley III; Van der Knaap Syndrome
Introduction
The Van der Knaap Syndrome, also known as Megalencephalic Leukoencephalopathy with Subcortical Cyst (MLC) is a genetic damage of the brain white matter that begins in infancy. The disease has autosomal recessive inheritance and was first described in 1995 [1,2]. MLC is a rare neurodegenerative disease. Although there is no evidence-based data on the incidence, it is higher in countries such as Turkey and India where consanguineous marriages are more common [1,3-6].
Genetic studies have associated MLC with the 22q chromosome [7]. The disorder was found to be associated with the mutation of two genes. These are MLC1 and HEPACAM (Named GLIALCAM) [8]. The MLC1 gene mutation is seen in 70-80% of the patients [9,10]. An infant with MCL is healthy at birth but macrocephaly develops without neurological findings during the first year of life and white matter is seen to emit abnormal signals and expand on cerebral Magnetic Resonance Imaging (MRI) [1,4,11,12]. The head growth rate starts to normalize after the first year. Macrocephaly can be borderline in certain patients but it is usually a striking finding [1,4,13]. Cerebellar ataxia and to a smaller extent spasticity may develop in these children as a slowly progressing process over a couple of years [1,4,11,12]. Motion disorders such as dystonia or ataxia can also be seen due to extrapyramidal involvement [13]. The onset of independent walking is usually slightly delayed compared to normal peers. The ability to walk may be lost at advanced ages. Walking is not stable. Patients may have to use an independent wheelchair, usually starting at adolescence. The cognitive developments may initially be normal or mildly delayed [1,4,11,12]. The cognitive damage becomes more evident in time but the communication and social skills can remain undamaged [1,4]. Autism is seen in about half of the patients with cognitive disorder [4]. Another finding that may affect the expected survival and motor development of these children is epileptic seizures [13-15]. Epileptic seizures that can be controlled with drugs are seen in almost all these children [1]. Deaths due to epileptic seizures before the age of 15 years has been reported in the literature but most patients live to the age of 40 or 50 [5,16-18].
Movement disorders, spasticity, cerebellar ataxia, progressive severe motor and functional disorders with age, and cognitive and similar problems indicate that the child should be supported with a physiotherapy and rehabilitation program. We did not find any study on the physiotherapy and rehabilitation of these patients. The purpose of this study was to determine the long-term results of physiotherapy and rehabilitation in a case with MLC.
Case Presentation
A 12-month-old male patient was referred to the Paediatric Rehabilitation Unit of the Physiotherapy and Rehabilitation Department at the Hacettepe University Faculty of Health Sciences in Ankara, Turkey. The parents reported fourth-degree consanguinity. The gestational age was 37 weeks and the birth weight was 1750 grams. He had been delivered with emergency caesarean section because of meconium aspiration syndrome and had stayed at the neonatal intensive care unit for 18 days. Intrauterine growth retardation had been found on the 30th week of pregnancy and the growth at birth was shown to be consistent with the 32nd week of pregnancy. Deep tendon reflexes were prominent in both lower extremities. Electroencephalography at 1 year of age showed moderate epileptic signs at the central areas in the left hemisphere and treatment was started with Luminaletten. Cerebral MRI at the same age revealed widened appearance due to an atrophic pattern at the ventricular system and cerebral sulci in supratentorial sections. Signal changes that were hypo intense on T1-weighted images and hyper intense on T2 weighted images were present in the cortical corticomedullary distance and marked in both temporal lobes. Cystic changed that were more evident in the temporal regions were observed. The findings were reported to be consistent with Van der Knaap disease and glutaricaciduria. Bilateral moderate sensorineural hearing loss was detected again at the same period, at the age of 1. Behavioural responses were observed to speaking voices at 65-70 dB. According to the gross motor development stages evaluation, prone head control gained at the 6th months, supine head control at the 9th months, sitting independently at the 13th months, crawling at the 18th months and independent walking at the 30th months. Multi-level Botulinum Toxin (Botox) administration was performed to bilateral low extremities at the age of 3 years. A second Botox administration was performed only to bilateral gastrocnemius muscles at the age of 4 years.
Method
Physiotherapy and rehabilitation evaluation methods
1- Patient demographic information (prenatal, natal, postnatal history, EEG, MRI, hearing test results): These were obtained from hospital records, the medical chart and the family.
2- Bayley Scales of Infant and Toddler Development - Third Edition (Bayley-III): This is a norm-referenced scale/test to measure the developmental level of children aged 1-42 months. It consists of five different development scale sections named Cognitive, Language, Social - Emotional, Motor and Adaptive Behaviour. The Bayley III scales include growth scores that can be calculated to follow-up the progress of the individual over time. The standard score is between 40 and 160. The score is accepted as extremely good for 130 points and above, good for 120-129, high average for 110-119, normal for 90-109 normal, low average for 80-89, borderline for 70-79 and very low for 69 and below for all motor, cognitive and language development. The recommended cut-off score is between 100 and 115 [19].
3- Gross Motor Function Measurement (GMFM-88): These measurements have been developed to evaluate the gross motor functions in children with Cerebral Palsy (CP). The measurements are based on five sections consisting of Supine-Prone, Sitting, Crawling, Standing up and Walking-Climbing stairs. Each item is scored between 0 and 3. 0 means the child cannot do the movement, 1 means initial part of the movement to <10%is done, 2 means the part of the movement from 10% to <100% is done, and 3 means the entire movement is done. The score of each section is then calculated using the 'child's score/maximum score x 100%) formula. The total score is obtained by dividing the total for the five sections into five. 100% is the best expected and 0% is the worst score [20].
4- Tonus Evaluation: The severity of the spasticity was evaluated according to the Modified Ashworth Scale (MAS) [21]. MAS scores for spasticity over 6 points between 0 and 4 as 0, 1, 1+, 2, 3, and 4. "0" means no increase in muscle tonus and "4" means a rigid influence on motion [22].
Treatment
Neurodevelopmental treatment: The physiotherapy program administered by a paediatric physical therapist began when our case was 12 months old and continued 3 days per week for 36 months according to the Neurodevelopmental Treatment (NDT) approach. Each treatment session lasted 60 minutes. The home program given to the family was checked and updated in the last 15 minutes. The treatment program implemented is shown in Table 1.
Performer Activity
12 months
18 months
24 months
36 months
48 months
(Follow-up)
Supine
?
?
?
?
?
Weight transfer to the upper body and shoulder girdle with the elongation of the back extensors.
Taking the bridge position with knee extension
?
?
?
?
?
Prone
Reaching for the exercise ball with both hands
?
?
?
?
?
Sitting
Strengthening the abdominal and back muscles using upper extremity handling with an extensor pattern
?
?
?
?
?
Standing
Weight transfer to extremities in the aided standing posture
?
?
?
?
?
Weight transfer with body elongation in front of the mirror
?
?
?
?
?
Taking the half above knee position and transfering to standing up when sitting
?
?
?
?
?
Lying on a narrow support surface
?
?
?
?
?
Stretching on a single foot while aided, throwing a ball to different directions on both sides
?
?
?
?
?
Walking
Aided side walking
?
?
?
?
?
Walking sideways with obstacles
?
?
?
?
?
Walking backwards
?
?
?
?
?
Walking on a narrow support surface
?
?
?
?
?
Going up and down the stairs
?
?
?
?
?
Walking on the treadmill
?
?
?
?
?
? Activity that continues to be implemented in the treatment program
? Activity that has been discontinued in the treatment program.
Table 1: The Neurodevelopmental Treatment-Based Physiotherapy and Rehabilitation Program.
The objectives of the current Bobath approach is to observe, analyse, interpret the existing performance in the child and then to evaluate the potential of the child and finally to achieve the maximum independent level within the limitations [23,24].
The main purposes of Neurodevelopmental Treatment (NDT) are as follows:
- To improve the body flexion-extension muscle balance
- To increase the stabilization of shoulder girdle
- To strengthen deep back muscles
- To strengthen the gluteus medius and maximus.
- To elongate the thoracic and lumbar muscles
- To develop postural control
- To strengthen anti-gravity muscles
- To provide alignment
- To improve walking.
Results
The results of the Bayley-III and GMFM evaluations in the 12th, 18th, 24th, 36th and 48th months are presented in Table 2 and Table 3. The cognitive, motor and language development of our case was "extremely low", at a level far below his peers according to the Bayley- III scores. The GMFM scores gradually increased with age. The change in GMFM scores over time is shown in Figure 1.
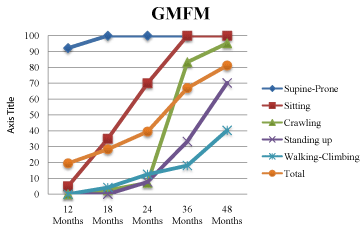
Figure 1: The change of GMFM scores over time.
Bayley-III
12 Months
18 Months
24 Months
36 Months
48 Months
Motor
61*
61*
64*
58*
55*
Cognitive
65*
55*
60*
55*
55*
Language
62*
50*
56*
53*
50*
*******130 points and over extremely superior,
******120-129 superior,
*****110-119 high average,
****90-109 normal,
***80-89 low average,
**70-79 border,
*69 and below extremely low
The recommended mean score is between 100 and 115.
Table 2: Bayley III Cognitive-Motor-Language Results.
Supine-Prone (%)
Sitting (%)
Crawling (%)
Standing (%)
Walking - Climbing (%)
Total (%)
12 Months
92.16%
5%
0%
0%
0%
19.43%
18 Months
100%
35%
9.5%
3.6%
0%
29.62%
24 Months
100%
70%
38.09%
7.69%
12.5%
45.65%
36 Months
100%
100%
83.33%
33.3%
18.05%
66.94%
48 Months
100%
100%
95.23%
70.2%
40.2%7
81.14%
Table 3: The scores of GMFM in a case with MLC.
Bilateral hip flexor, adductor, hamstring and gastrocnemius muscle tonus values are shown in Table 4. The tonus was normal when 12 months old but increased with age.
MAS
Hip Flexors
Hip Adductors
Hamstring
Gastrocnemius
Right
Left
Right
Left
Right
Left
Right
Left
12 Months
0
0
0
0
0
0
0
0
18 Months
0
0
0
0
0
1
0
1
24 Months
1
1
1
1
1
1
1
2
36 Months
1
1
2
2
1
2
2
3
48 Months
0
1
2
2
0
1
2
3
Table 4: The Modified Ashworth Scale Results.
Discussion
The children diagnosed with MLC reported in the literature have a history of normal birth. Neurological disorders such as ataxia, spasticity, dystonia emerge in these children in time and result in a progressive severe motor disorder [1,4,11,12]. Our MLC case was included in the NDT-based physiotherapy program following the preliminary evaluations performed in the 12th month. We did not come across any study on a physiotherapy and rehabilitation program in an MCL case in the literature. We therefore believe the data from this case report can provide guidance, as it is the first to show the effectiveness of physiotherapy in this rare but important disorder that is thought to have a relative higher prevalence in our country.
NDT is commonly used in different clinical pictures causing sensory-motor disorders and especially in children with CP [25,26]. It has been used in the treatment of children with CP for many years and constitutes the foundation of such physiotherapy, especially in Europe, but there are various theories on the degree to which it is evidence based [27,28]. NDT has been reported to be clinically important but a complete statistical evaluation of its results has not been provided [27]. It is unfortunately not possible to distinguish how much the child who is growing and developing is influenced by the process and how effective the treatment is. New ideas have therefore been incorporated into the NDT concept over time and NDT therapists have included evidence-based implementations such as motor learning, familyoriented applications, Botulinum Toxin, strengthening training, constraint-induced movement therapy, treadmill, and Kinesio Taping into their intervention programs [29]. The role of the family within the therapy has become more important [26,30]. The key person in the implementation of NDT is the therapist. The therapist guides the intervention process and helps the family in making decisions [31]. NDT therapists inform the family about the needs or strengths of their babies [32]. They teach the family handling, positioning, and carrying methods and nutritional information appropriate for the child in addition to the exercises that should be done at home [31,32]. Knox et al [33] studied 15 paediatric CP patients with a mean age of 7 years 4 months included in a Local Therapy and NDT program. The patients attended 3 treatment sessions per week, each lasting 75 minutes. The children received local therapy for the first 12 weeks of the study that lasted 18 weeks and participated in the NDT program for a total of 6 weeks between the 7th and 12th weeks. The minimum 3 targets were identified together with the family to be compatible with GMFM before starting NDT. The total GMFM targeted score was 48.77% before NDT implementation and 55.80% afterwards. The initial total GMFM score was 58.56% and increased to 61.20% after six weeks of treatment. Comparison of the total and targeted GMFM showed that the score increases following the first 6 weeks when only local therapy was used were less than those after NDT. Tsorlakis et al [34] evaluated the gross motor development of the children who received NDT treatment two times a week and five times a week with GMFM before and after the treatment in two groups with CP. A total of 34 children with a mean age of 7 years and 3 months were included in the study. The session duration was 50 minutes and the program lasted for 16 weeks. There was no significant difference between the initial evaluations of 2 groups. There was a significant change in the GMFM results for both groups after treatment. When the results of the two groups were compared, the group that had received more intensive NDT treatment showed a larger increase compared to the other group as expected. Kara et al [35] examined the efficiency of an NDT-based physiotherapy program in a child with Angelman syndrome. Gross motor function was evaluated with GMFM and the gross motor performance with GMPM (Gross Motor Performance Measurement). They showed that the scores increased together with age and physiotherapy. The total GMFM scores we used to evaluate gross motor function in our case also increased with age and physiotherapy as expected. However, the part of this increase due to the physiotherapy and the part related to growth and development is not known. Kara et al [36] used the Bayley-III and GMFM as to evaluate the effectiveness of early physiotherapy and the post-treatment results in a case with Incontinentia Pigmenti (IP) where motormental retardation, epilepsy, microcephaly, spasticity, paralysis and ataxia were present due to CNS involvement [37,38]. Although there was an improvement in cognitive and motor development according to the Bayley-III evaluation, motor and cognitive retardation became more evident with growth. Bayley-III motor, language and cognitive scores of our case are included in very low average with advancing time. We believe that it is possible that the child could not catch up with his peers regarding neurodevelopmental development as cognitive, language and motor skills necessary became more difficult with growth.
Conclusion
In conclusion, the gross motor function of our case increased with NDT-based physiotherapy but the cognitive, language and motor development skills were behind his peers. This study is especially important for clinicians as it provides the long-term results of physiotherapy and rehabilitation programs in a child with the progressive disorder MLC who gained functionality within certain limits but could not reach the functionality level of his peers. A longterm physiotherapy and rehabilitation program was effective for gross motor development in our patients with Van der Knaap Syndrome. However, studies with more cases where objective evaluation tools are used are required.
References
- van der Knaap MS, Barth PG, Stroink H, van Nieuwenhuizen O, Arts WF, Hoogenraad F, et al. Leukoencephalopathy with swelling and a discrepantly mild clinical course in eight children. Ann Neurol. 1995; 37: 324-334.
- Van der Knaap MS. Forget About "van der Knaap syndrome," forget about glycine. AJNR Am J Neuroradiol. 2003; 24: 1030.
- Yis U, Scheper GC, Uran N, Unalp A, Cakmakci H, Hiz-Kurul S, et al. Two cases with megalencephalic leukoencephalopathy with subcortical cysts and MLC1 mutations in the Turkish population. Turk J Pediatr. 2010; 52: 179-183.
- Singhal BS, Gursahani RD, Udani VP, Biniwale AA. Megalencephalic leukodystrophy in an Asian Indian ethnic group. Pediatr Neurol. 1996; 14: 291-296.
- Singhal BS. Leukodystrophies: Indian scenario. Indian J Pediatr. 2005; 72: 315-318.
- Gorospe JR, Singhal BS, Kainu T, Wu F, Stephan D, Trent J, et al. Indian Agarwal megalencephalic leukodystrophy with cysts is caused by a common MLC1 mutation. Neurology. 2004; 62: 878-882.
- Topcu M, Gartioux C, Ribierre F, Yalcinkaya C, Tokus E, Oztekin N, et al. Vacuoliting megalencephalic leukoencephalopathy with subcortical cysts, mapped to chromosome 22qtel. Am J Hum Genet. 2000; 66: 733-739.
- Van der Knaap MS, Scheper GC. Megalencephalicleukoencephalopathy with subcortical cysts. Pagon RA, Bird TD, Dolan CR, Stephens K, editors. Gene Reviews. In: Seattle: University of Washington, 1993.
- Patrono C, Di Giacinto G, Eymard-Pierre E, Santorelli FM, Rodriguez D, De Stefano N, et al. Genetic heterogeneity of megalencephalic leukoencephalopathy and subcortical cysts. Neurology. 2003; 61: 534-537.
- Blattner R, Von Moers A, Leegwater PA, Hanefeld FA, Van Der Knaap MS, Kohler W. Clinical and genetic heterogeneity in megalencephalic leukoencephalopathy with subcortical cysts (MLC). Neuropediatrics. 2003; 34: 215-218.
- Goutieres F, Boulloche J, Bourgeois M, Aicardi J. Leukoencephalopathy, megalencephaly, and mild clinical course. A recently individualized familial leukodystrophy. Report on five new cases. J Child Neurol. 1996; 11: 439-44.
- Topcu M, Saatci I, Topcuoglu MA, Kose G, Kunak B. Megalencephaly and leukodystrophy with mild clinical course: a report on 12 new cases. Brain Dev. 1998; 20: 142-153.
- Ben-Zeev B, Gross V, Kushnir T, Shalev R, Hoffman C, Shinar Y, et al. Vacuolating megalencephalic leukoencephalopathy in 12 Israeli patients. J Child Neurol. 2001; 16: 93-99.
- Riel-Romero RM, Smith CD, Pettigrew AL. Megalencephalic leukoencephalopathy with subcortical cysts in two siblings owing to two novel mutations: case reports and review of the literature. J Child Neurol. 2005; 20: 230-234.
- Bugiani M, Moroni I, Bizzi A, Nardocci N, Bettecken T, Gartner J, et al. Consciousness disturbances in megalencephalic leukoencephalopathy with subcortical cysts. Neuropediatrics. 2003; 34: 211-214.
- Saijo H, Nakayama H, Ezoe T, Araki K, Sone S, Hamaguchi H, et al. A case of megalencephalic leukoencephalopathy with subcortical cysts (van der Knaap disease): molecular genetic study. Brain Dev. 2003; 25: 362-366.
- Singhal BS, Gorospe JR, Naidu S. Megalencephalic leukoencephalopathy with subcortical cysts. J Child Neurol. 2003; 18: 646-652.
- Pascual-Castroviejo I, van der Knaap MS, Pronk JC, Garcia-Segura JM, Gutierrez-Molina M, Pascual-Pascual SI. Vacuolating megalencephalic leukoencephalopathy: 24 year follow-up of two siblings. Neurologia. 2005; 20: 33-40.
- Bayley N. Bayley Scales of infant and toddler development. 3rd edn. San Antonio, Texas, USA: Harcourt Assessment, 2006.
- Russell DJ, Rosenbaum PL, Cadman DT, Gowland C, Hardy S, Jarvis S. The gross motor function measure: a means to evaluate the effects of physical therapy. Dev Med Child Neurol. 1989; 31: 341-352.
- Bohannon RW, Smith MB. Interrater reliability of a modified Ashworth scale of muscle spasticity. Phys Ther. 1987; 67: 206-207.
- Ashworth B. Preliminary trial of carisoprodol in multiple sclerosis. Practitioner. 1964; 192: 540-542.
- Kerem Gunel M. The view on pediatric rehabilitation with the title of cerebral palsy from the perspective of a physiotherapist, ActaOrtopaedica Et TraumatologiaTurcica. 2009; 43: 173-181.
- Bly L. A historical and current view of the basis of NDT. Pediatric Physical Therapy. 1991; 3: 131-135.
- Livanelioglu A, KeremGunel M. Serebralpalsidefizyoterapi. Ankara: Hacettepe Universitesi. OzbekMatbasi. 2009.
- Mayston MJ. People with cerebral palsy: effects of and perspectives for therapy. Neural Plast. 2001; 8: 51-69.
- Kerem M, Livanelioglu A. Effects of neurodevelopment therapy on motor development in children with cerebral palsy. FizyoterRehabil. 2003; 13: 117-23.
- Bobath K, Bobath B. The neuro-developmental treatment. Scrutton D, editor. In: Management of the Motor Disorders of Children with Cerebral Palsy. Philadephia: JB Lippincott. 1984; 6-18.
- Butler C, Darrah J. Effects of neurodevelopmental treatment (NDT) for cerebral palsy: an AACPDM evidence report. Dev Med Child Neurol. 2001; 43: 778-790.
- Finnie NR. Handling the Young Child with Cerebral Palsy at Home. Oxford: Butterworth-Heinemann. 1996.
- Dirks T, Hadders-Algra M. The role of the family in intervention of infants at high risk of cerebral palsy: a systematic analysis. Dev Med Child Neurol. 2011; 53 Suppl 4: 62-67.
- Bly L. Baby Treatment based on NDT Principles. Austin, TX: PRO-ED. 1999.
- Knox V, Evans AL. Evaluation of the functional effects of a course of Bobath therapy in children with cerebral palsy: a preliminary study. Dev Med Child Neurol. 2002; 44: 447-460.
- Tsorlakis N, Evaggelinou C, Grouios G, Tsorbatzoudis C. Effect of intensive neurodevelopmental treatment in gross motor function of children with cerebral palsy. Dev Med Child Neurol. 2004; 46: 740-745.
- Kara OK, Mutlu A, Gunel MK, Haliloglu G. Do the physiotherapy results make us happy in a case with 'happy puppet' (Angelman) syndrome? BMJ Case Rep. 2010; 2010.
- Kara OK, Mutlu A, Gunel MK. The results of early physiotherapy on a child with incontinentiapigmenti with encephalocele. BMJ Case Rep. 2010.
- Berlin AL, Paller AS, Chan LS. Incontinentia pigmenti: a review and update on the molecular basis of pathophysiology. J Am Acad Dermatol. 2002; 47: 169-187.
- Welbury TA, Welbury RR. Incontinentia pigmenti (Bloch-Sulzberger syndrome): report of case. ASDC J Dent Child. 1999; 66: 213-215, 155.