
Case Report
Austin J Clin Case Rep. 2023; 10(4): 1288.
A Fatal Infection Caused by Dolosigranulum Pigrum in a Patient with Lung Cancer: A Case Report
Bracci S1; Di Capua B2; Zinicola T1*; Bellieni A2; Giordano R3; Bertolini R4; Nicolì A4; Colloca GF1; Gambacorta MA1,4; Valentini V1,4
1UOC di Radioterapia Oncologica, Dipartimento Diagnostica per Immagini, Radioterapia Oncologica ed Ematologia, Fondazione Policlinico Universitario Agostino Gemelli IRCCS, Italy
2Centro di Eccellenza Oncologia Radioterapica e Medica e Radiologia, Fatebenefratelli Isola Tiberina, Gemelli Isola, Italy
3Dipartimento di Scienze Geriatriche e Ortopediche, Università Cattolica del Sacro Cuore, Italy
4Dipartimento di Scienze Radiologiche ed Ematologiche, Università Cattolica del Sacro Cuore, Italy
*Corresponding author: Zinicola T Department: UOC di Radioterapia Oncologica, Dipartimento Diagnostica per Immagini, Radioterapia Oncologica ed Ematologia, Fondazione Policlinico Universitario Agostino Gemelli IRCCS, 00168 Roma, Italy Tel: +39 327 876 5116 E-mail: tiziano.zinicola@guest.policlinicogemelli.it
Received: May 09, 2023 Accepted: June 03, 2023 Published: June 10, 2023
Abstract
Dolosogranulum Pigrum is a rare gram-positive catalase-negative coccus and a commensal organism residing in the upper respiratory tract that can cause fatal opportunistic infections in immunocompromised patients.
In this report, we describe a case of a 62-year-old male patient affected by metastatic lung cancer treated with chemotherapy, radiotherapy and immunotherapy, who had dyspnea and positive blood cultures for the bacterium Dolosigranulum Pigrum, associated with bilateral interstitial pneumonia, initially confused with pneumonia related to SARS-CoV-2 infection. Although Dolosigranulum Pigrum appears to be very susceptible to current antibiotics, as ampicillin, ceftriaxone, levofloxacin, and vancomycin, it can cause serious infections in humans, particularly in patients with baseline pulmonary diseases, such as lung cancer, cystic fibrosis, and severe chronic obstructive pulmonary disease, until the patient’s death.
Keywords: Dolosigranulum pigrum; Lung cancer; SARS-CoV-2; Personalized medicine; Case report
Introduction
Lung cancer is a heterogeneous disease. Despite advances in understanding treatment options for lung cancer, the prognosis is dismal, and it remains the leading cause of cancer death. The goal of therapy for patients depends on the stage of the disease but the multimodality therapy, included surgery, radiotherapy, chemotherapy and immunotherapy remains the standard [1,2].
In this period of COVID-19 pandemic, it is fundamental to identify particular diseases with a similar clinical presentation to Covid 19 in order to make a proper differential diagnosis.
In this report we describe the case of a 62-year-old male patient affected by metastatic lung cancer with a rare lung infection, initially confused with Covid-19 infection.
He had a positive blood culture for the bacterium Dolosigranulum Pigrum, a rare gram-positive catalase-negative coccus, associated with bilateral interstitial pneumonia, which initially often presents features of pneumonia related to SARS-CoV-2 infection [3].
Case Presentation
The patient’s history (a 62-year-old male, non-smoker) dates back to June 2020 when he was diagnosed with metastatic lung adenocarcinoma. Immunohistochemistry showed that tumor cells were positive for TTF-1 and negative for p63; ROS1 and ALK were rearranged, Ki67 was 70%, PDL-1 was 1-2% molecular analysis showed KRAS gene mutations, BRAF gene wild type, and EGFR gene wild type.
Between September 2020 and December 2020, he received four chemotherapy cycles with Pemetrexed, Cisplatin, and Pembrolizumab. In December 2020, he underwent palliative radiotherapy on left humeral metastases. The radiotherapy was delivered with a 3-D conformal technique (3D-CRT) for a total dose of 20 Gy in 5 fractions. In January 2021, he underwent palliative radiotherapy on lumbar vertebra L2 and the seventh right rib. The radiotherapy was delivered with a 3-D conformal technique (3D-CRT) for a total dose of 30 Gy in 10 fractions.
During last radiotherapy, the patient presented to the emergency room for progressively increased pain, and he was admitted to our inpatient radiotherapy oncology ward of Fondazione Policlinico Universitario Agostino Gemelli in Rome.
Performance status was lacking. He reported severe neuropathic pain (VAS 8-9) partially controlled with opioid analgesics and neuromodulator drugs. During the physical examination, the patient's lungs' auscultation revealed decreased breath sounds over the whole lung field. Bilateral crepitations, similar to velcro crackles, were audible during slow, deep breaths, predominant during inspiration, sometimes associated with exhalation crackles.
Initial vital signs were: blood pressure 110/70mmHg, heart rate 75 beats/min, and oxygen saturation 95% in room air. Laboratory exams showed a white blood cell count of 5.15×109/L with 4.77×109/L neutrophils, 0.21×109/L lymphocytes, and 5.15×109/L 2.8 monocytes. Hemoglobin was 12.3g/dl and platelets 189×109/L. The C Reaction Protein (CRP) level was 158.2mg/L, while procalcitonin was normal.
During hospitalization, the patient developed fever (>38°C), chills, shortness of breath with oxygen desaturation 89% and need for oxygen support (Fraction of inspired O2 FiO2 24%), cough without expectoration, and complained of pain in his chest wall over the area of the lung cancer and at the level of the left shoulder. A chest X-ray confirmed the primary tumor's lesion located in the left lobe and showed multifocal lung consolidations and bilateral Ground-Glass Opacities (GGO). Because of suggestive clinical signs and bilateral opacities on the chest X-rays, on suspicion of COVID-19 infection, the patient was placed in isolation.
The probability of developing severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infections in cancer patients appears to be higher than in the general population, mainly because they are immunosuppressed patients both due to neoplastic disease and treatments they receive; furthermore, GGO and consolidation in COVID-19 could mimic radiotherapy- or chemotherapy and immunotherapy-associated pneumonitis. [4]
The double Reverse Transcription-Polymerase Chain Rreaction (RT-PCR) results from the nasopharyngeal swab samples within 72 hours remained negative. Therefore, this patient's differential diagnosis included: other infections (e.g., viral disease, bacterial infection, mycotic infection), immunotherapy-induced pneumonitis, chemoradiation-induced pneumonitis, progressive lung cancer (Table 1).
Differential diagnosis
1
Infections: viral disease, bacterial infection, mycotic infection
2
Immunotherapy-induced pneumonitis
3
Chemoradiation-induced pneumonitis
4
Progressive lung cancer
Table 1: Possible differential diagnoses of respiratory failure in relation to the clinical presentation of our patient.
Test for Human Immunodeficiency Virus (HIV) was negative, but quantitative cytomegalovirus DNA resulted positive (4263 UI/mL).
Blood culture was performed, and empirical antibiotic therapy was started; it was associated with Caspofungin therapy due to Beta d Glucan's positive result and with intravenous Valganciclovir that represents the standard treatment for cytomegalovirus infection.
A chest CT scan was performed and showed extensive areas of parenchymal thickening with air bronchogram in the upper lobes and subpleural ground-glass-like changes in the adjacent parenchyma, associated with modest bilateral pleural effusion. In Figure 1, two images of the CT scan of the chest are shown.
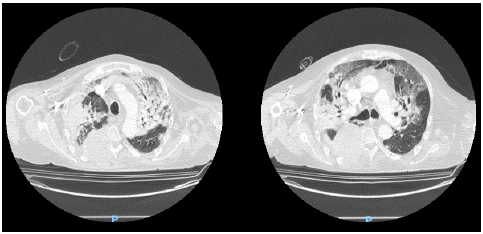
Figure 1: Two images of chest CT scan showing parenchymal thickening and subpleural ground-glass-like areas.
In the following days, the patient developed an acute exacerbation of dyspnea with severe acute respiratory distress syndrome and showed deteriorating conditions with values of oxygen saturation of less than 80% with FiO2 60%; arterial blood gas analysis showed a pH7.49; pCO2 31.7mmHg; pO2: 52.2mmHg; HCO3-: 26mmol/l; SpO2 90%, so he required ventilator support with BLB oxygen mask. Seven days into admission, blood cultures revealed Dolosigranulum Pigrum, a rare gram-positive opportunistic pathogen. Following the antibiogram evaluation, we started a massive intravenous antibiotic therapy with Vancomycin, Meropenem, and Trimethoprim-sulfamethoxazole. A repeated chest X-ray showed progression of bilateral lung architectural distortion, consolidative opacities, and ground-glass opacities.
Despite comprehensive treatment, including antibiotics, assisted oxygenation, and other supportive care, the clinical conditions of the patients and his respiratory state worsened with a progressive reduction of peripheral capillary oxygen saturation (SpO2 45% in BLB), up to the evolution in the form of multi-organ failure which led to the death of the patient after three weeks from the admission. Figure 2 summarizes the timeline of the patient's history from cancer diagnosis to death.
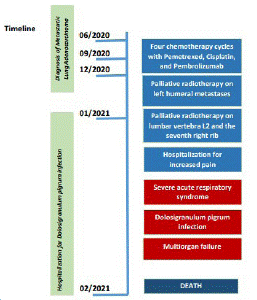
Figure 2: Timeline.
Discussion
Dolosigranulum Pigrum has emerged in different studies of the human Upper Respiratory Tract (URT) microbiota, often associated with Corynebacterium species, as potentially protective against colonization by S. aureus and S. pneumonia. [6,7] Despite D. Pigrum can cause eye infections, sepsis, nosocomial pneumonia, and ventilator-associated pneumonia, there are very few reports of D. Pigrum in association with human disease. [8] The pathogen it's frequently identified in human nasal microbiota. [9,10] However, few case reports to date have documented a variety of severe infections. [11-15] most cases of severe infections reported in the literature concern patients with previous serious diseases but none of these led to patient death. All this suggests that D. Pigrum is an emerging pathogen that mainly targets immunocompromised patients with lung diseases and can result in the severest cases in death.
In conclusion, Dolosigranulum Pigrum is a commensal organism resident in the upper respiratory tracts and appears to be very susceptible to current antibiotics, as ampicillin, ceftriaxone, levofloxacin, and vancomycin. In patients with baseline pulmonary diseases, such as lung cancer, cystic fibrosis, and severe chronic obstructive pulmonary disease, D. Pigrum can cause serious infections until the patient's death.
More research is needed to understand better the impact of this bacterium in terms of severity of the infection based on the individual patient's profile to identify risk factors that predict the disease.
References
- Gadgeel SM, Ramalingam SS, Kalemkerian GP. Treatment of lung cancer. Radiol Clin North Am. 2012; 50: 961-74.
- Bade BC, Dela Cruz CS. Lung Cancer 2020: Epidemiology, Etiology, and Prevention. Clin Chest Med. 2020; 41: 1-24.
- Sherret J, Gajjar B, Ibrahim L, Mohamed Ahmed A, Panta UR. Dolosigranulum pigrum: Predicting Severity of Infection. Cureus. 2020; 12: e9770.
- Dingemans AC, Soo RA, Jazieh AR, Rice SJ, Kim YT, et al. Treatment Guidance for Patients With Lung Cancer During the Coronavirus 2019 Pandemic. J Thorac Oncol. 2020; 15: 1119-36.
- Aguirre M, Morrison D, Cookson B, Gay F, Collins M. Phenotypic and phylogenetic characterization of some Gemella-like organisms from human infections: description of Dolosigranulum pigrum gen. nov., sp. nov. Journal of applied bacteriology. 1993; 75: 608-12.
- Laufer AS, Metlay JP, Gent JF, Fennie KP, Kong Y, et al. Microbial communities of the upper respiratory tract and otitis media in children. mBio. 2011; 2: e00245-10.
- Brugger SD, Eslami SM, Pettigrew MM, Escapa IF, Henke MT, et al. Dolosigranulum pigrum Cooperation and Competition in Human Nasal Microbiota. mSphere. 2020; 5: e00852-20.
- Laclaire L, Facklam R. Antimicrobial susceptibility and clinical sources of Dolosigranulum pigrum cultures. Antimicrob Agents Chemother. 2000; 44: 2001-3.
- Wos-Oxley ML, Plumeier I, Von Eiff C, Taudien S, Platzer M, et al. A poke into the diversity and associations within human anterior nare microbial communities. The ISME journal. 2010; 4: 839-51.
- De Boeck I, Wittouck S, Wuyts S, Oerlemans EF, Van den Broek MF, et al. Comparing the healthy nose and nasopharynx microbiota reveals continuity as well as niche-specificity. Frontiers in microbiology. 2017; 8: 2372.
- Hall GS, Gordon S, Schroeder S, Smith K, Anthony K, et al. Case of synovitis potentially caused by Dolosigranulum pigrum. J Clin Microbiol. 2001; 39: 1202-3.
- Lin JC, Hou SJ, Huang LU, Sun JR, Chang WK, et al. Acute cholecystitis accompanied by acute pancreatitis potentially caused by Dolosigranulum pigrum. J Clin Microbiol. 2006; 44: 2298-9.
- Lecuyer H, Audibert J, Bobigny A, Eckert C, Janniere-Nartey C, et al. Dolosigranulum pigrum causing nosocomial pneumonia and septicemia. J Clin Microbiol. 2007; 45: 3474-5.
- Bittar F, Richet H, Dubus J-C, Reynaud-Gaubert M, Stremler N, et al. Molecular detection of multiple emerging pathogens in sputa from cystic fibrosis patients. PloS one. 2008; 3: e2908.
- Sampo M, Ghazouani O, Cadiou D, Trichet E, Hoffart L, et al. Dolosigranulum pigrum keratitis: a three-case series. BMC Ophthalmol. 2013; 13: 31.