
Case Report
Austin J Clin Case Rep. 2023; 10(6): 1298.
Unveiling a Rare Complication: Gemcitabine-Induced Cardiomyopathy in Ovarian Serous Adenocarcinoma
Muhammad Nabeel¹*; Khubaib Ali²
¹Department of Clinical Oncology Pharmacy, Cancer Care Hospital & Research Centre, Lahore, Punjab, Pakistan
²Akhtar Saeed College of Pharmaceutical Sciences, Bahria Town, Lahore, Punjab, Pakistan
*Corresponding author: Muhammad Nabeel Department of Clinical Oncology Pharmacy, Cancer Care Hospital & Research Centre, Lahore, Punjab, Pakistan Tel: +92-321-4873-648 Email: nabeelsheikh26@gmail.com
Received: July 17, 2023 Accepted: August 28, 2023 Published: September 04, 2023
Abstract
Gemcitabine is a nucleoside analogue and pyrimidine antimetabolite authorized for the treatment of ovarian cancer. It is generally considered safe and well-tolerated, with only a few reported cases of cardiac adverse effects. However, we present a case of gemcitabine-induced dilated cardiomyopathy in a 33-year-old female receiving gemcitabine as second-line therapy for ovarian serous adenocarcinoma. The patient had no history of hypertension or significant cardiac issues. She presented with clinical symptoms, laboratory abnormalities, and imaging findings consistent with congestive cardiac failure, along with a Left Ventricular Ejection Fraction (LVEF) of 25-30%. Gemcitabine administration was immediately discontinued, and treatment with Furosemide, ACE inhibitors, and Beta-blocker agents was initiated. Subsequently, the patient’s condition improved, with the resolution of symptoms and normalization of cardiac findings upon discontinuation of gemcitabine. This is the first reported case demonstrating objective evidence of gemcitabine-induced dilated cardiomyopathy in a patient with ovarian serous adenocarcinoma without a significant cardiac history. Although rare, it is crucial to promptly diagnose gemcitabine-induced cardiomyopathy to initiate appropriate management protocols.
Keywords: Gemcitabine; Cardio-oncology; Cardiomyopathy; Cardiotoxicity; Ovarian cancer
Introduction
Cancer is rapidly emerging as the second leading cause of mortality worldwide, primarily due to the alarming increase in cancer incidence and treatment complications. The overall annual cancer cases are on a significant rise, leading to increased morbidity and mortality. Despite advancements in cancer treatment, the lack of effective curative therapies, along with adverse treatment effects, drug resistance, and tumor recurrence, contribute to these challenges [1]. Gemcitabine, a pyrimidine antimetabolite, and cytidine analogue, is widely used in the treatment of various solid organ malignancies [2,3]. It acts by inhibiting ribonucleotide reductase and DNA repair, thereby suppressing DNA synthesis. It is commonly employed in the management of lung, pancreatic, bladder, breast, ovarian, and bile duct carcinomas, as well as lymphomas and uterine sarcomas, either alone or in combination with other therapies [4]. Gemcitabine is often utilized as an adjuvant chemotherapy agent [5], due to its relatively lower toxicity profile compared to other anticancer drugs, making it well-tolerated and considered safe [6]. While myelosuppression is the most common side effect, gemcitabine has been associated with gastrointestinal disturbances (nausea, vomiting, and diarrhea) and abnormalities in liver and renal function tests [7]. Moreover, rare Adverse Drug Reactions (ADRs) have been reported, including thrombotic microangiopathy [8], interstitial pneumonitis [9], arterial fibrillation [10], and Capillary Leak Syndrome (CLS) [11]. Although gemcitabine has not shown a substantial risk of cardiotoxicity in phase 1 and 2 clinical studies, there have been a few reported cases of acute Myocardial Infarction (AMI) and arrhythmias associated with its widespread clinical use [2].
Here, we present a case of a 33-year-old female with stage 3 ovarian serous adenocarcinoma who developed dilated cardiomyopathy following gemcitabine chemotherapy for ovarian cancer treatment.
Case Presentation
In February 2022, a 33-year-old woman with no comorbidities presented with a two-year history of lower abdominal pain. A Computerized Tomography (CT) scan of the abdomen and pelvis revealed a significant malignant-looking pelvic mass originating from the right adnexa and extending into the lower abdomen. An ultrasound-guided biopsy of an anterior abdominal wall nodule was performed to obtain tissue for diagnosis. Cancer biomarkers mentioned in Table 1 were found to be positive, indicating the presence of cancer.
Further confirmation of the primary tumor was obtained through a Magnetic Resonance Imaging (MRI) scan, which diagnosed the patient with ovarian serous adenocarcinoma. She was initiated on neo-adjuvant chemotherapy using the carboplatin/paclitaxel (CARB+PAC) protocol to reduce the size of the cancer and prepare for subsequent debulking surgery.
Therapeutic Intervention
After completing 7 cycles of chemotherapy, an echocardiogram (Echo) was performed (Figure 1), revealing an increased thickness of the Left Ventricle (LV) wall without any abnormalities in systolic function. The Ejection Fraction (EF) was determined to be more than 55%. Exploratory laparotomy and bilateral ureteric stenting were performed, but the tumor was found to be inoperable due to its size, location, and involvement of nearby organs. Based on these findings, the oncology department recommended a change in the chemotherapy protocol from carboplatin/paclitaxel (CARB+PAC) to gemcitabine (1000 mg/m2 IV on days 1, 8, 15 on a 28-day cycle for six cycles) as the tumor appeared to be resistant to platinum-based chemotherapy.
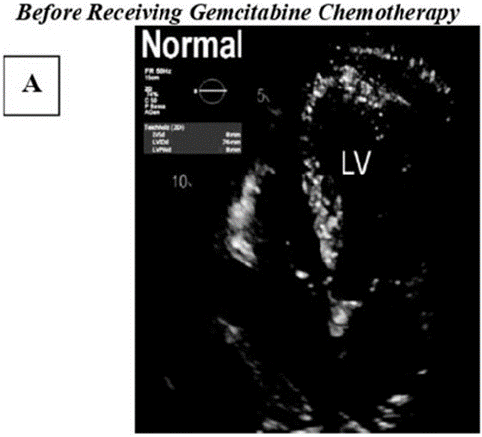
Figure A: Illustrates a 2D normal echo image show casing the typical echocardiographic findings observed in a healthy heart. The image displays clearly defined cardiac chambers, strucuturally intact valves, and normal patterns of blood flow.
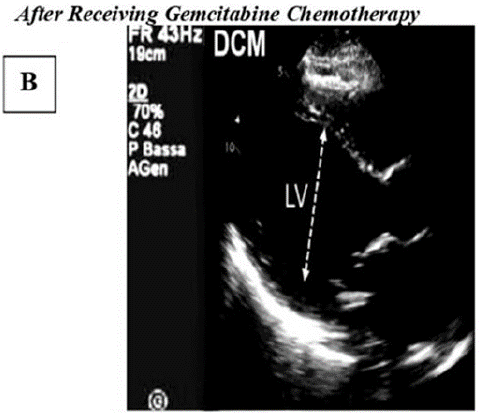
Figure B: Highlights echocardiographic characteristics associated with dilated cardiomyopathy (DCM). The left ventricle demonstrates significant enlargement and reduced contractile strength, leading to compromised pumping ability.
Diagnostic Evaluations
During the examination, the patient exhibited bilateral pedal edema, elevated Jugular Venous Pressure (JVP), a tachycardic pulse rate of 120 beats per minute, and a blood pressure reading of 175/134 mmHg. Crepitation in the chest was also noted. A chest X-ray revealed a mild to moderate pleural effusion on the right side. The patient's symptoms were attributed to fluid overload, leading to the administration of Intravenous (IV) furosemide, which provided partial relief. The following day, an echocardiogram (Echo) was conducted, revealing a moderately Dilated Left Ventricle (DLV), impaired overall left ventricular systolic function with a Left Ventricular Ejection Fraction (LVEF) ranging between 25% and 30%, and a moderate, widespread pericardial effusion (maximum depth of 1.2 cm). Additionally, the patient's Troponin-I level was measured at 0.095 ng/ml. These laboratory findings deviated from the standard values, as outlined in Table 2.
Emergency Management
As the patient's cardiac symptoms deteriorated, the medical team focused on managing her edematous condition. Treatment began with a Furosemide infusion to alleviate fluid overload. A nitrate infusion was also initiated at a rate of 1 to 3 mg per hour. Subsequently, the patient was transferred to the High-Dependency care Unit (HDU) for continuous cardiac monitoring. In addition to diuretics, an Angiotensin-Converting Enzyme Inhibitor (ACEI) was prescribed to address the patient's high blood pressure.
Gemcitabine-Induced Cardiomyopathy
Although the precise etiology of dilated cardiomyopathy in our patient remained unclear, gemcitabine was considered the most probable culprit. The timing of symptom manifestation correlated with the initiation of gemcitabine chemotherapy. Moreover, the absence of risk factors for ischemic cardiomyopathy, the lack of a prior history of Coronary Artery Disease (CAD), and the presence of global hypokinesia on the Echo report all supported the diagnosis of gemcitabine-induced cardiomyopathy.
Discussion
We reported a case of ovarian cancer, specifically ovarian serous adenocarcinoma, the patient had no cardiac comorbidities but suffered dilated cardiomyopathy after receiving gemcitabine chemo protocol for the treatment of ovarian cancer. This dilated cardiomyopathy was thought to be an adverse effect of gemcitabine monotherapy.
Several Anti-cancer antibiotics (doxorubicin, daunorubicin, anthracyclines, mitomycin, bleomycin, etc.) and antimetabolites (5-fluorouracil (5-FU)) are notorious for their cardiotoxic adverse effects. Furthermore, certain modern biological therapies, such as trastuzumab and rituximab, are recognized for potentially cardiotoxic side effects. However, cardiotoxicity related to gemcitabine has not been widely reported in the literature before [12].
Several investigations showed that gemcitabine monotherapy had no significant cardiotoxic adverse effects. According to certain clinical trial data, cardiac arrhythmias and exudative pericarditis were identified in phase 1 gemcitabine trials with a significant drop in Left Ventricular Ejection Fraction (LVEF) [13]. While other studies revealed no evidence of significant cardiotoxicity in phase 2 clinical trials of gemcitabine monotherapy [14]. This indicates that gemcitabine is a safe medicine with relatively no risk of cardiomyopathy; yet, after widespread clinical use of gemcitabine, certain outcomes linked to cardiomyopathy were seen [15], as we reported. Numerous studies support our clinical case by demonstrating; Gemcitabine was linked to Myocardial Infarction (MI), Supra ventricular Arrhythmia (SVA), Heart Failure (HF), and pericardial disorders. Clinical investigations have also identified the same cardiotoxicity signals from gemcitabine [3].
A recent analysis of the French Pharmacovigilance Database revealed a possible relation between dilated cardiomyopathy and gemcitabine treatment [16]. Based on different studies of the medical literature, gemcitabine cardiotoxicity can be categorized into acute and chronic toxicities [17], as well as other acute cardiac events such as myocardial infarction, angina, arrhythmias, and arterial fibrillation. Cardiomyopathy emerges after a few months, to years, of gemcitabine therapy. The exact mechanisms of heart dysfunction leading to cardiomyopathy are dubious [18,19]. The majority of patients who developed gemcitabine-induced Cardiomyopathy (CMP) in phase 1 and 2 clinical trials had underlying Coronary Artery Disease (CAD). Even though our patient had no known CAD or CAD risk factors, it is plausible that she experienced a past silent episode (a fixed inferior wall defect on MPI). On the other hand, the fixed inferior wall defect does not explain the markedly reduced Left Ventricular (LV) systolic function, and widespread hypokinesia revealed on 2D Echo. Given that, her heart failure symptoms began shortly after starting gemcitabine, we suspected gemcitabine cardiotoxicity as the cause of his CMP [20,21].
Another study revealed that Gemcitabine has also been related to the development of exudative pericardial effusions. Four different cases of symptomatic and hemodynamically severe pericardial effusions necessitating drainage were presented with the same complication as in our case [22].
The key message of our case report is that the administration of more than one chemotherapeutic drug, each with a unique mechanism of action, can result in significant cardiotoxicity while being administered in multiple doses. In the era of combination chemotherapy, which employs many cytotoxic agents, it is critical to understand that an interaction between the effects of various treatments may exist, leading to cardiac dysfunction that is either unexpected or worse than predicted.
Conclusions
In conclusion, our patient initially underwent the carboplatin/paclitaxel (CARB+PAC) treatment protocol for ovarian serous adenocarcinoma, and upon completion of the chemotherapy regimen, she exhibited increased Left Ventricle (LV) wall thickness with normal systolic function. However, upon switching to gemcitabine monotherapy, she developed dilated cardiomyopathy. Existing literature supports the occurrence of gemcitabine-induced cardiomyopathy, and it is plausible that the prior use of CARB+PAC followed by gemcitabine could contribute to the development of cardiomyopathy. While this situation is rare, our case report provides compelling evidence of gemcitabine-induced cardiomyopathy in a patient who had previously received a CARB+PAC protocol.
Possible Aspects
Based on our clinical observations, we have identified gemcitabine as a potential inducer of cardiotoxicity, particularly cardiomyopathy. In future clinical oncology practice, it is crucial to closely monitor and observe patients who have received gemcitabine following the CARB+PAC protocol for any signs of gemcitabine-induced cardiotoxicity, specifically cardiomyopathy, as demonstrated in our case. Furthermore, it is essential to share this information with other cancer treatment facilities to gather data on similar patients and to alert them to the potential adverse effects. Caution should be exercised when administering gemcitabine, especially in patients with pre-existing cardiovascular issues or a family history of cardiovascular problems, if the occurrence of cardiotoxicity is confirmed with this treatment modality.
Author Statements
Acknowledgment
We would like to acknowledge Cancer Care Hospital & Research Centre, Lahore (CCH&RC) to provide the data for this case report. Both Authors contributed equally to the manuscript, and should be considered as co-first authors.
References
- Fiza Ur Rehman. W, Syeda Sohaila Naz, Kifayat Ullah Shah. Anticancer therapeutics: a brief account on wide refinements; 2020.
- Khan MF. SG, RBaEJ. Gemcitabine-induced cardiomyopathy: a case report and review of the liter. Available from: https://jhoonline.biomedcentral.com/articles/10.1186/s13045-022-01263-x; 2014.
- Hilmi M, Ederhy S, Waintraub X, Funck-Brentano C, Cohen A, Vozy A, et al. Cardiotoxicity associated with gemcitabine: literature review and a pharmacovigilance study. Pharmaceuticals (Basel). 2020; 13: 325.
- Eli Lilly and Company, GEMZAR (Gemcitabine) [package insert]. U.S. Food and Drug Administration Website; 2020. Revised May 2014.
- [cited Mar 5, 2022] Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/020509s077lbl.pdf.
- Nasreen Shaikh FI, Sardar M, Shah A. Gemcitabine induced cardiomyopathy; 2019.
- Syed S, Fatima S. A case of gemcitabine-induced cardiomyopathy. Chest. 2021; 160.
- Aapro MS, Martin C, Hatty S. Gemcitabine--a safety review. Anti Cancer Drugs. 1998; 9: 191-201.
- Humphreys BD, Sharman JP, Henderson JM, Clark JW, Marks PW, Rennke HG, et al. Gemcitabine-associated thrombotic microangiopathy. Cancer. 2004; 100: 2664-70.
- Roychowdhury DF, Cassidy CA, Peterson P, Arning M. A report on serious pulmonary toxicity associated with gemcitabine-based therapy. Investig New Drugs. 2002; 20: 311-5.
- Santini D, Tonini G, Abbate A, Di Cosimo S, Gravante G, Vincenzi B, et al. Gemcitabine-induced atrial fibrillation: a hitherto unreported manifestation of drug toxicity. Ann Oncol. 2000; 11: 479-81.
- Mertz P, Lebrun-Vignes B, Salem JE, Arnaud L. Characterizing drug-induced capillary leak syndromes using the World Health Organization VigiBase. J Allergy Clin Immunol. 2019; 143: 433-6.
- Alam S, Illo C, Ma YT, Punia P. Gemcitabine-induced cardiotoxicity in patients receiving adjuvant chemotherapy for pancreatic cancer: A case series. Case Rep Oncol. 2018; 11: 221-7.
- Mattiucci GC, Ippolito E, D’Agostino GR, Alfieri S, Antinori A, Crucitti A, et al. Long-term analysis of gemcitabine-based chemoradiation after surgical resection for pancreatic adenocarcinoma. Ann Surg Oncol. 2013; 20: 423-9.
- Mohebali D, Matos J, Chang JD. Gemcitabine induced cardiomyopathy: a case of multiple hit cardiotoxicity. ESC Heart Fail. 2017; 4: 71-4.
- Adão R, de Keulenaer G, Leite-Moreira A, Brás-Silva C. Cardiotoxicity associated with cancer therapy: pathophysiology and prevention strategies. Rev Port Cardiol. 2013; 32: 395-409.
- Ferrari D, Carbone C, Codecà C, Fumagalli L, Gilardi L, Marussi D, et al. Gemcitabine and atrial fibrillation: a rare manifestation of chemotherapy toxicity. Anti Cancer Drugs. 2006; 17: 359-61.
- Vogl DT, Glatstein E, Carver JR, Schuster SJ, Stadtmauer EA, Luger S, et al. Gemcitabine-induced pericardial effusion and tamponade after unblocked cardiac irradiation. Leuk Lymphoma. 2005; 46: 1313-20.
- Storniolo AM, Allerheiligen SR, Pearce HL. Preclinical, pharmacologic, and phase I studies of gemcitabine. Semin Oncol. 1997; 24: S7-2.
- Montastruc G, Favreliere S, Sommet A, Pathak A, Lapeyre-Mestre M, Perault-Pochat MC, et al. Drugs and dilated cardiomyopathies: a case/noncase study in the French pharmacovigilance Database. Br J Clin Pharmacol. 2010; 69: 287-94.
- Melissa K, Accordino AIN, Hershman DL. Cardiac effects of anticancer therapy in the elderly; 2014.