
Case Series
Austin J Clin Case Rep. 2024; 11(1): 1313.
Severe Idiopathic Pulmonary Haemosiderosis – A Rare Cause For Acute Respiratory Distress Syndrome
André P Becker, MD1,2*; Torben M Rixecker, MD1,2; Guy Danziger1,2; Adriana Nistor, MD3; Sebastian Mang, MD1,2; Robert Bals, Prof1,2; Philipp M Lepper, Prof1,2
¹Department of Internal Medicine V – Pneumology, Allergology and Intensive Care Medicine, University Hospital of Saarland, Homburg, Germany
²Interdisciplinary COVID-19-Center, University Medical Centre, Saarland University, Homburg/Saar, Germany
³Department of Pathology, University Medical Centre, Saarland University, Homburg/Saar, Germany
*Corresponding author: André P Becker, M.D Department of Internal Medicine V – Pneumology and Critical Care Medicine, ECLS Center Saar, University Hospital of Saarland, Kirrbergerstr. 1, 66421 Homburg, Germany. Tel: +49(0)6841-1615211 Email: andre.becker@uks.eu
Received: January 10, 2024 Accepted: February 10, 2024 Published: February 17, 2024
Abstract
Background: Idiopathic Pulmonary Haemosiderosis (IPH) is a very rare disease that can cause recurring haemoptysis and acute respiratory failure. Classical symptoms consist of haemoptysis, iron-deficiency anaemia and radiologic pulmonary infiltrates [1,2] The pathogenesis of the disease is not fully elucidated, yet an immune pathological component that may be responsive to immunmodulatory therapy seems to be existing [7-9].
Case Summary: We report two cases of IPH with severe exacerbation, as two young patients were transferred to our intensive care unit. In the first case, the cause of respiratory failure was primarily unknown, while the history of the patient revealed two similar previous episodes, at that time considered as acute distress syndrome due to viral infection. This time, diagnosis of idiopathic pulmonary hemosiderosis was made. The fulminant presentation causing acute respiratory failure due to diffuse alveolar haemorrhage made invasive ventilation and extracorporal membrane oxygenation necessary to bridge the time until a diagnosis was made and a high dose of steroids improved the situation of the patient. The patient was successfully weaned from ECMO and IMV and was discharged finally. In the second case, IPH was already known to the patient and due to acute exacerbation and haemoptysis a high-flow oxygen therapy and non-invasive ventilation was necessary. Similar to the first case, high dose of steroid improved the situation of the patient, making oxygen therapy no longer necessary.
Conclusion: These cases suggest an immune modulatory therapeutic component in idiopathic pulmonary haemosiderosis and underlines the reversibility of in this disease. Further, the first case emphasizes the benefit of ECMO even in situations of excessive bleeding.
Case 1 Presentation
Written informed consent was obtained for publication of this case report according to standard of CARE guidelines. In March 2021, a 14 years old girl (39 kg) was transferred to our ECLS-center due to respiratory failure of unknown cause. Three days before admission to the hospital, the girl suffered from acute fatigue, fever and hemoptysis. As symptoms aggravated and dyspnea occurred, the girl was brought to an external hospital. On admission, the patient was tachypneic, tachycardic and had a peripheral oxygen-saturation of spO2 79%. Informations about the initial arterial blood gas analysis were not available, however oxygen demand was 6 L/min via nasal cannula to reach a peripheral oxygen-saturation >90 %. Test result for SARS-CoV-2 was negative. The patient had a rapidly increasing
oxygen demand to nasal high-flow therapy and was thus transferred to the pediatric centre of our hospital. On admission, the patient had to be intubated and mechanically ventilated due to hypoxaemia (spO2 50 %) and respiratory acidosis (apCO2 81 mmHg, pH 7,23). Oxygenation index during invasive ventilation was 60 mmHg with a Positive-End-Expiratory Pressure (PEEP) of 12 cmH2O. Compliance of the lung was decreased below 10 ml/cmH2O resulting in hypercapnia and respiratory acidosis with a pH<7.1. Further the patient showed shock symptomatic as there was hemodynamic support with vasopressors (0.3 μg/kg BW norepinephrine) and slightly elevated arterial lactate level (3 mmol/l). Lactate level normalized after transfusion of 2 PRBC (initial Hb 4.6 g/dl). Echocardiography ruled out a cardiac origin of hemodynamic instability and X-ray scan showed bilateral infiltration dominating in the upper lung regions (figure 1). Bronchoscopy was performed, whereby diffuse alveolar haemorrhage was suspected. Consequently, the working diagnosis was severe acute respiratory distress syndrome due to diffuse alveolar haemorrhage of unknown origin. To facilitate protective ventilation, veno-venous ECMO with minimal anticoagulation was initiated. A blood flow of 4 L/min and a gas flow of 4 lpm was established for sufficient oxygenation with an arterial pO2 of 60 – 80 mmHg with normalized pCO2 and pH. Laboratory findings presented in Table 1 revealed anaemia due to a combination of iron deficiency and bleeding, there were neither signs of kidney or liver failure nor signs of bleeding diathesis or haemolysis. There were slight elevated parameters of humoral inflammation, therefore broad empiric antibiosis with Meropenem/Clarithromycin and Linezolid war initiated. Microbiological and virological assessment gave later no relevant information that led to a diagnosis. A chest CT-scan showed diffuse ground-glass opacification with partially consolidation and positive bronchopneumogram in both lungs (Figure 1).
Laboratory findings
Day 1
(reference)Hemoglobin [g/dL]
5.3(12–16)
MCV [fL]
64(80–99)
MCH [pg]
18(27–33)
Reticulocytes [%]
1.87(0,8–4.1)
Leukocytes [G/L]
10.2(4.5–11.4)
Thrombocytes [G/L]
161(154–442)
CRP [mg/L]
77.3(0–5)
Interleukin-6 [pg/mL]
18.5(<7)
Creatinin [mg/dL]
0.53(0.57–0.87)
Bilirubin [mg/dL]
4.0(<1.2)
PCT [ng/mL]
1.02(<0.5)
INR
1.32(0.85–1.15)
aPTT [s]
20(21–34)
Haptoglobin [mg/dL]
134(38–205)
Ferritin [ng/mL]
1839(10–20)
sTfR [mg/L]
32.9(1,7–4,1)
MCV: Mean Corpuscular Volume; MCH: Mean Corpuscular Haemoglobin; CRP: C-Reactive Protein; PCT: Procalcitonin; INR: International Normalized Ratio; aPTT: Activated Partial Thromboplastin Time; sTfR: Soluble Transferrin Receptor
Table 1: Initial laboratory findings of patient (case one) on day 1.
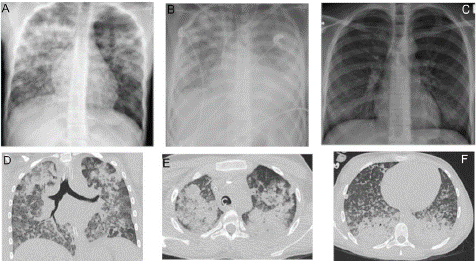
Figure 1: Case 1, chest x-ray and thoracic CT-scan.
Chest x-ray on day 1, day 3 (after vvECMO implantation) and day 14, left to right Thoracic CT-scan on day 2. CT scan shows diffuse ground-glass opacification with partially consolidation and positive bronchopneumogram in both lungs. Predominant regions are the upper and lower, dorsal parts of the lungs.
Medical history of the girl revealed two similar episodes of lung failure in 2018 with mechanical ventilation in an external hospital. Back then, an infection with human influenza virus was considered to be responsible for the lung failure. Further, anaemia as a result of an iron deficiency was known as a comorbidity. Third-party history of the girl gave no hints of taken medication or toxic expositions.
In consequence of the fulminant diffuse haemorrhage with following lung failure, an autoimmunological process was suspected. In addition to laboratory diagnostics, a cryobiopsy in areas with GGO was carried out during vvECMO therapy, no complication occurred during biopsy. As infectious causes were either ruled out or unlikely and samples for histopathological or serological tests were taken but would need time to establish a final diagnosis, we decided to treat her with 1g of methylprednisolone over 3 days with a maintenance dose of 1 mg/kg body weight.
Within three days, the condition of the girl improved rapidly. There was no more alveolar bleeding and weaning from mechanical ventilation was quickly possible. The patient could be extubated successfully three days after start of steroid treatment, veno-venous ECMO was continued until a diagnosis was made. Autoimmunological laboratory findings were not suggestive for vasculitis or any other common autoimmune processes. In broncho-alveolar lavage, 92% alveolar macrophages, free haemosiderin and haemosiderin-bearing macrophages were identified. The pathological assessment showed highly siderophagia of alveolar macrophages with no signs of vasculitis or granuloma (Figure 2).
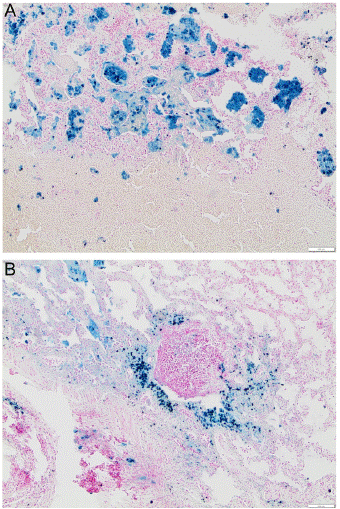
Figure 2: Case 1, cryo biopsy, lung tissue. Haemosiderin-laden macrophages (blue) (above). Sedimentation of haemosiderin-laden macrophages (blue) and erythrocytes in the interstitium and
bronchus (below).
In synopsis of the findings the diagnosis of an idiopathic pulmonary haemosiderosis was made as an exclusion diagnosis. In this case however, essential facts varied from the usual, rising suspicion of the unusual during times of exceptional situations.
Eventually, vvECMO could be removed and the girl was discharged to the pediatric NCU and was later able to be discharged home with outpatient connection. Steroid treatment was reduced within four weeks to a maintenance dose of 5 mg per day, X-ray improved and the girl’s lung function was re-established. No bleeding or respiratory problems occurred within the twelve weeks observation period.
Case 2 Presentation
Written informed consent was obtained for publication of this case report according to standard of CARE guidelines.
In March 2022, a young 18 years old woman was transferred to our emergency centre due to cough and dyspnoea. Idiopathic pulmonary haemosiderosis was already stated in the medical history of the patient. Anamnestic investigation revealed that the patient suffered from reduced performance and fatigue since weeks and had acute dyspnoea at admission to the hospital. Initial spO2 was 70% with tachypnoea while there were clinical signs of infection or haemoptysis. Point-of-care diagnostics revealed hypoxaemia (pO2 54 mmHg, pCO2 32 mmHg, pH 7.38) with anaemia (Hb 5, 1g/dl), with no signs of lactatemia (<2 mmol/l). Supply of oxygen via nasal cannula was initiated but had to be escalated to high-flow therapy and later non-invasive ventilation with FiO2 of 100 % and PEEP of 6 mmHg due to worsening of hypoxaemia. Laboratory diagnostics (Table 2) showing slightly elevated humoral inflammation with anaemia. The x-ray diagnostics are displayed in “Supplemental Figure 1”, revealing bilateral infiltration of the lungs suspicious for acute respiratory distress syndrome after exclusion of cardiac reasons for infiltration.
Laboratory findings
Day 1
(reference)Hemoglobin [g/dL]
5.1(12–16)
MCV [fL]
62(80–99)
MCH [pg]
18(27–33)
Reticulocytes [%]
4.79(0,8–4.1)
Leukocytes [G/L]
16.5(4.5–11.4)
Thrombocytes [G/L]
388(154–442)
CRP [mg/L]
22.9(0–5)
Creatinin [mg/dL]
0.57(0.57–0.87)
Bilirubin [mg/dL]
0.6(<1.2)
PCT [ng/mL]
0.19(<0.5)
INR
1.04(0.85–1.15)
aPTT [s]
22(21–34)
Ferritin [ng/mL]
199(10–20)
sTfR [mg/L]
25.8(1,7–4,1)
MCV: Mean Corpuscular Volume; MCH: Mean Corpuscular Haemoglobin; CRP: C-Reactive Protein; PCT: Procalcitonin; INR: International Normalized Ratio; aPTT: Activated Partial Thromboplastin Time; sTfR: Soluble Transferrin Receptor
Table 2: Initial laboratory findings of patient (case two) on day 1.
Considering medical history of pulmonary haemosiderosis, early steroid therapy was initiated with 1g of methylprednisolone over 3 days with maintenance of bodyweight 1 mg/kg for 2 weeks. In addition, we started empiric antibiosis with ampicilline/sulbactame and clarithromycine for 5 days to treat possible bacterial infection. Due to lack of haemoptysis, we didn’t perform bronchoscopy. Diagnosis was acute exacerbation of IPH. Within 5-6 days of high flow oxygen therapy with intermitting non-invasive ventilation the patient recovered so that oxygen supply via nasal cannula (3–5 lpm) was possible and the patient could be transferred to the normal care unit. Further x-ray showed progressive benefit of anti-inflammatory therapy (Supplemental Figure 1). After 12 days the patient was discharged home with outpatient connection without oxygen supply. Over an observation period of 4 weeks no further respiratory symptomatic.
Discussion
We report two rare cases of severe idiopathic pulmonary haemosiderosis with ARDS and for one case extracorporeal membrane oxygenation support. According to the literature, most of the patients presented with intermittent episodes of haemoptysis [1-3] that are followed by a symptom-free intervall. Classical symptoms consist of haemoptysis, iron-deficiency anaemia and radiologic pulmonary infiltrates [1,2].
Epidemiological data is inconsistent due to the rarity of this disease. However, most patients are diagnosed in pediatric age [2]. In the first case, the patient had two similar episodes of respiratory failure with haemoptysis requiring invasive ventilation in the past. At that time, a misdiagnosis was made, which nearly caused the death of the patient. Previously, the detection of viral DNA in a respiratory specimen (influenza) led to another plausible diagnosis, but the correct pathomechanism remained elusive. This time, a fulminant clinical course required maximal intensive care treatment with vvECMO to sustain the patient’s life. The leading symptom was diffuse pulmonary haemorrhage.
The differential diagnosis for this symptom is complex and needs broad and interdisciplinary work-up. The usual causes and triggers for pulmonary haemorrhage [4,5] could be ruled out by laboratory and microbiological/virological diagnostics. Therefore, histopathological assessment became necessary. While broncho-alveolar lavage and the tissue samples showed high percentage of haemosiderin-laden macrophages (siderophages) and other causes of alveolar haemorrhage could be ruled out, the diagnosis of idiopathic pulmonary haemosiderosis could be made [6]. In the second case, the patient was already diagnosed with IPH, but had respiratory failure due to acute exacerbation of IPH.
The pathogenesis of the disease is not fully elucidated, yet an immune pathological component that may be responsive to immunmodulatory therapy seems to be existing [7-9]. Because of the rapid improvement and the stable clinical course after induction of steroid therapy, it is likely that immune mechanisms sensitive to steroids are also relevant in this case. Few weeks since the first occurance of the symptoms both patients were in stable condition under steroid maintainance therapy.
In summary three findings could be made presenting this case. First, it suggests an immune modulatory therapeutic component in idiopathic pulmonary haemosiderosis and underlines the reversibility of severe diffuse alveolar haemorrhage in this disease. Second, the first case emphasizes the benefit of ECMO even in situations of excessive bleeding. Third, it points out that even if the diagnostic path leads to a plausible connection between cause and effect, it might not be the correct causal origin.
References
- Chen XY, Sun JM, Huang XJ. Idiopathic pulmonary hemosiderosis in adults: review of cases reported in the latest 15 years. Clin Respir J. 2017; 11: 677-681.
- Ioachimescu OC, Sieber S, Kotch A. Idiopathic pulmonary haemosiderosis revisited. Eur Respir J. 2004; 24: 162-70.
- Ibrahem RE, Arasaretnam A, Ordidge K, Vedelago J, Toy R. Case Report of Idiopathic Pulmonary Haemosiderosis in a Child with recurrent chest infections. Journal of Radiology Case Reports. 2011; 5: 30-5.
- Susarla SC, Fan LL. Diffuse alveolar hemorrhage syndromes in children. Current Opinion in Pediatrics. 2007; 19: 314-20.
- Lara AR, Schwarz MI. Diffuse Alveolar Hemorrhage. Chest. 2010; 137: 1164-71.
- Castellazzi L, Patria MF, Frati G, Esposito AA, Esposito S. Idiopathic pulmonary haemosiderosis in paediatric patients: how to make an early diagnosis. Italian Journal of Pediatrics. 2016; 42: 86.
- Ahmed M, Raj D, Kumar A, Kumar A. Anaemia and respiratory failure in a child: can it be idiopathic pulmonary haemosiderosis? BMJ Case Reports. 2017; 2017: bcr-2017-219431.
- Saha BK. Idiopathic pulmonary hemosiderosis: A state of the art review. Respiratory Medicine. 2021; 176: 106234.
- Yang CT, Chiang BL, Wang LC. Aggressive corticosteroid treatment in childhood idiopathic pulmonary hemosiderosis with better outcome. J Formos Med Assoc. 2021; 120: 838-846.