
Case Report
Austin J Clin Case Rep. 2024; 11(1): 1316.
Brain Abscess in a Patient with Congenital Cardiopathy in Association with COVID-19: A Case Report
Martha Lilia Tena-Suck1*; Graciela Cardenas, MD, PhD2; Jose Luis Soto-Hernandez, MD2; Alma Ortiz-Plata, PhD3; Sergio Moreno, MD4
1Departamento de Neuropatología, Instituto Nacional de Neurología y Neurocirugía, Ciudad de México
2Departamento de infectología, Instituto Nacional de Neurología y Neurocirugía, Ciudad de México
3Laboratorio de Neuropatología Experimental. Instituto Nacional de Neurología y Neurocirugía, Ciudad de México
4Servicio de Neurocirugía. Instituto Nacional de Neurología y Neurocirugía, Mexico
*Corresponding author: Martha Lilia Tena-Suck Departamento de Neuropatología, Instituto Nacional de Neurología y Neurocirugía, Ciudad de México. Email: mltenasuck@gmail.com
Received: February 02, 2024 Accepted: March 14, 2024 Published: March 21, 2024
Abbreviasions: BA: Brain Abscess; CNS: Central Nervous System; COVID19: Sars-Cov2 Virus; CBF: Cerebrospinal Fluid; ECs: Endothelial Cells; ACE2: Co-Infection; ACE2: Angiotensin Converting Enzyme 2; RAS eeceptor: Renin-Angiotensin System (RAS); PCR: Polymerase Chain Reaction; IHQ: Immunohistochemistry; ISH: In Situ Hybridization; EM: Electron Microscopy; CRP: C-Reactive Protein.
Introduction
Brain Abscess (BA) is usually produced by relating infection following sinusitis or middle-ear or tooth infections. They may arise spontaneously, or like as, a consequence of contiguous focus of infection spreading straight to the adjacent CNS, specific risk factors like as, intravenous drug use, congenital cardiac defects, infective endocarditis or immunosuppression, etc [1].
SARS-CoV-2 infection (COVID-19) primarily disturbs the respiratory system, other organs including the brain can be involved. It is unclear how much SARS-CoV-2 infection contributes to the incidence of stroke given co-morbidities in the affected patient population [2]. Bacterial infections have also been reported in half of COVID-19 hospitalized patients like as; pneumonia, sepsis, as well as, abscesses formation [3]. However, the coinfection of the SARS-CoV-2 with other microorganisms, can deep the difficulties of diagnosis, treatment, prognosis and even intensification the disease and mortality [4]. Co-pathogens included bacteria, such as Streptococcus pneumoniae, Staphylococcus aureus, Klebsiella pneumoniae, Mycoplasma pneumoniae, Chlamydia pneumonia, Legionella pneumophila and Acinetobacter baumannii; Candida species and Aspergillus flavus; and viruses such as influenza, rhinovirus/enterovirus, parainfluenza, metapneumovirus, influenza B virus, and human immunodeficiency virus [9]. The coinfection between different microorganisms and SARS-COV-2 is a serious problem in this pandemic. The factors that contribute in helping virus proliferation and invasive fungal or bacterial infections including cell-mediated immunity, associated immunocompromised conditions and treatment rules that slows down immune mechanisms [4]. The extensive use of antibiotics in early diagnosis of SARS-CoV-2 infection. However, it is certain which is due to the severity of the disease and the coinfection can increase the mortality [5].
The common neuropathological findings described; including acute encephalomyelitis [5], lymphoid inflammation, acute hypoxic-ischemic changes, astrogliosis, acute/subacute brain infarcts, spontaneous hemorrhage, and microthrombi, cerebrovascular disease and stroke [5-7], etc.
The aim of this word was a case report a 46- year- old man with congenital heart disease as comorbidity which presented a Streptococcus abscess associated to COVID-19 coinfection,
Case Description
46-year-old man, was undergoing cardiac surgery with septum closure at 3-year-old. He begins his actual condition 15 days before admission with intense right frontal headache, nauseas, 3 days later presented weakness of the right hemibody as well as loss of vision of the left eye. On physical exploration, the patient was cachectic, jaundiced, awake, well oriented, the campimetry with left bitemporal hemianopia, dysdiadochokinesia and left hypoparesis, gait with laterapulsion. A simple skull CT scan and MRI were taken showing right occipital tumors with a volume effect with a diagnosis of brain abscess (Figure 1a, 1b and 1c). A CT chest scan was performed, non-COVID-19 lesions were found (Figure 1d). Cerebrospinal Fluid (CBF) with pleocytosis. Laboratory results reported; hemoglobin of 12.1g/dL, plaqueless 120,000 u/mcL, leukocytes 2.3%, lymphocytes 5,000 cells/mcL, C-reactive protein of 9.3mg/dl, fibrinogen 493mg/dl y dimer D fraction of 6.23μg/ml.
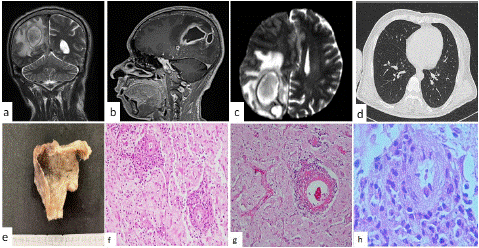
Figure 1: Brain abscess in a 46-year-old male (a) simple TAC imaging, (b) and (c) contra stated TAC imaging, (d) thoracic TAC imaging, (e) gross aspect of the surgical specimen with a saccular appearance. Histopathology in (f) Brain tissue shows variable degrees of necrosis, with inflammatory infiltrate of lymphocytes and polymorphonuclear cells (H&Ex400). (g) Fibrinoid necrosis and the small vessels presented fibrin thrombi (H&E x200). And in (h) observed the vessel wall was dissected producing glomeruloid-like appearance (H&E x400).
Steroids and analgesics are indicated for the time being for headache control. Surgery to drain the abscess was performed. The arachnoid at the parietal of the dorsal side showed a purulent appearance material, there is evidence of a tumor capsule which is punctured with a Yelko needle and was obtaining a greenish color purulent material in approximately 40 cc, the abscess capsule was dissected and completely resected. It is sent to pathology examination.
This purulent material cultured Streptococcus constellatus
The gross aspect was an ovoid tissue lesion, cystic in appearance, vs yellowish brown saccular, measuring 35x30x30mm. The external surface was dull opaque with a fibrinoid appearance. Surface of cut, the wall showed softened yellowish necrosis foci and the internal surface was reddish with a thickened hemorrhagic appearance with a granular surface (Figure 1e), with a variable average thickness that ranged from 3 to 5mm.
Histologically, brain tissue is identified that showed variable degrees of necrosis, with inflammatory infiltrate of lymphocytes and polymorphonuclear cells (Figure 1f). Blood vessels showed dense lymphoid infiltrate and polymorphonuclear leukocytes, in the Virchow-Robin’s space. This infiltrate dissected the vessel walls, with changes in the endothelial cells. It produced damage to the vascular lumen, with fibrinoid necrosis (Figure 1g). The small vessels presented fibrin thrombi. The vessel wall was dissected producing glomeruloid-like appearance (Figure 1h).
With Mason’s trichome stain, we observed extensive areas of fibrosis (Figure 2a), perivascular fibrosis with dissection of the vessels, and it became evident how inflammatory cells dissect the wall of the vessel. With staining for reticular fibers, the great production of fibers surrounding the blood vessels (Figure 2b), in the vessels of greater caliber they are made in the shape of onion cloth, but aneurysmal formations are made, in a close-up we observe reticular fibers dissect diffusely in the form of fishing nets (Figure 2c).
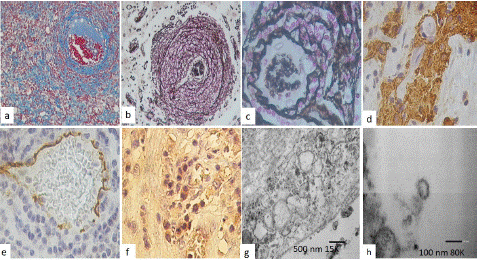
Figure 2: Histological features observed with Masson’s trichrome stain showed (a) extensive areas of fibrosis, perivascular fibrosis with dissection of the vessels (x200). Reticular fibers stain in (b) the great production of fibers surrounding the blood vessels, they are made in the shape of onion cloth, but aneurysmal formations (x200), and in (c) a close-up, showed reticular fibers dissect diffusely in the form of fishing nets (x400). (d) GFAP staining, observed areas of perivascular fibrous eschar (x400). (e) CD31 positive immunoreaction in blood vessels with swollen endocervical cells and in a loose form (x200), (f) virus spice protein showed immunoreaction in endothelial and in inflammatory cells (x200). Transmission Electron Microscopy analysis. Endothelial cells showed large pathologic vacuoles (arrows) in and some cytoplasmic lamellar bodies were found in (g). Free viral particles and visible along the plasma membrane were found in (h).
Immunohistochemical stain was performed showing a proliferation of T cells (CD3 +, CD8 +), focally CD20 +, increased macrophages (CD68 +++ and CD163 +), IL6 +, IL10 +, IL13 +, HIF1a +, TNFa +). GFAP staining, showed positive immunoexpression in perivascular fibrous eschar and hypertrophic perivascular astrocytes (Figure 2d). CD31 was positive in swollen endocervical cells and in a loose form, and in EC outside the vessel. ECs loss in larger vessels were observed (Figure 2e). The ACE2 was positive in endothelial cells and virus spike protein was positive in endothelial, as well as inflammatory cells (Figure 2f).
Electron microscopy analysis of CoV-2 infected cells shows destruction and extensive degeneration of brain tissue. Large vacuoles in endothelial and neuron cell were found in cytoplasm (Figure 2g). Viral particles were also observed in infected cells which induce stress on the endoplasmic reticulum, arranging themselves in lamellar bodies. Single viral particles resembled the “spherules” and viral particles visible along the plasma membrane were observed (Figure 2h).
Initially, a diagnosis of abscess was made in the process of resolution. And once the stains for COVID were performed, the diagnosis of brain abscess associated with COVD19 was made in an immunosuppressed cardiac patient, with negative COVID19 serology and no lung damage.
Discussion
COVID-19 predominantly affects the respiratory system and advanced age, cardiovascular disease, patients with chronic inflammatory conditions including hypertension, diabetes mellitus, and obesity, chronic respiratory disease, smoking, and cancer are risk factors may be disproportionally be affected by SARS-CoV-2 and experience a bigger severity of disease [6]. However, Bacterial co-infection or fungal infection in the ongoing pandemic of COVID-19 is associated with poor outcomes, mainly attacks hosts who have compromised immune systems [3,4]. Though, Microbial coinfection exacerbates the occurrence processes, development and prognosis of COVID-19, and the difficulties of clinical diagnosis and treatment [8]. Several authors believe that an area of infarction in the brain precedes the development of an abscess although this process alone has not been reported in congenital heart disease. The paradoxic emboli, as a result of thrombosis of the cerebral arteries. Especially in COVID-19 patients at high risk of mortality and brain abscess formation.
The virus is detectable by PCR, immunohistochemistry IHQ, In Situ Hybridization (ISH), RNA-Dependent Reverse transcriptase (RdRp) genes, identifying of virus in frozen or formalin-fixed paraffin-embedded brain tissue or Electron Microscopy (EM) [6]. COVID-19 RT-PCR testing may produce false-negative results in the initial phase of infection [7]. SARS-CoV-2 immunohistochemistry, using antibodies that recognize the viral Nucleocapsid (N) or spike (S) proteins, have been reported as negative in most attempted human cases of autopsy [7]. The use of immunostaining can be very useful, especially when there are not enough clinical data or when serological positivity for PCR has been confirmed; however, the lack of controls in most of these studies limits the interpretation of these findings. Finally, it is noteworthy that inflammation does not always coincide with the location of the virus, raising concerns that the virus may be evading the immune response in the CNS. SARS-CoV-2 has not been detected in Cerebrospinal Fluid (CBF) in the majority of patients tested [7].
Various pathophysiologic mechanisms of neurologic sequelae have been proposed, including injury to the vascular wall and epithelial cells, resulting in disruption of the blood brain barrier; hypoxic injury and prolonged period of ventilation [6,7]. The entry of SARS-CoV-2 into human cells is commonly supposed to be mediated by the communication and interaction between the spike protein with the Angiotensin Converting Enzyme 2 (ACE2) receptor, an important regulator of the Renin-Angiotensin System (RAS) [6]. ACE2 receptor–facilitated entry of the virus into neural tissue cells; and immune cell and injury is secondary to cytokine storm syndrome [1]. SARS-CoV-2–induced coagulopathy significantly contributes to the development of neurologic manifestations and brain injury in patients with COVID-19 disease [7].
The classical neuropathology findings of COVID19 viral CNS infections including; perivascular mononuclear cell cuffing, neuropil infiltration by polymorphonuclear leukocyte lymphocytic leptomeningitis and encephalitis, microglial nodules, perivascular lymphocytic cuffing, focal demyelination and viral inclusions [6-8]. Perivascular-activated microglia, macrophage infiltrates, and hypertrophic astrocytes are also seen [6-8].
Talaminti et al [10], reported 6 cases of primary bacterial spinal epidural abscess in association with COVID19 infection. That they were surgically managed over a 2-month period. Clinically none had evidence of septic infection. All cases were apyretic and never they presented signs of sepsis, and only leukopenia. In the 6 patients, the cultures identified methicillin-sensitive staphylococcus aureus. And they hypothesized that epidural abscess may develop because asymptomatic bacterial colonization coexisted with COVID19-induced damage to the endothelial cells and that this could favor retrograde spinal invasion at the corresponding level, vs a probable nosocomial infection [10].
Diagnosis of coronavirus particles by EM is challenging due to similar appearance normal cellular structures, which has shaped substantial controversy in the literature [12,13]. The EM changes that SARS-CoV-2 induces in infected cells, including; focused on the membranous replication organelles that support viral RNA fusion and, cytoplasmatic vacuoles, similar to endosomes on the meeting, and release of new virions, various distinct membrane alterations and multisystemic Cellular Tropism of SARS-CoV-2 have been described [12,13]. Double-membrane vesicles, convoluted membranes, while zippered endoplasmic reticulum [13].
CRP, and d-dimer (d-D) has been found as an important marker that changes significantly in severe patients with COVID-19, which serves as an initial marker of infection and inflammation in predicting the possibility of disease progression [14].
Conclusion
We present a rare case of difficult diagnosis not due to the fact of having a simple brain abscess associated with streptococcal infection, with COV- protein positive in some inflammatory cells and in endothelial cells and ACE2 was also positive in ECs, and CD8, Il6, Il10, Il20 and Il23 positive immunoreaction. And by EM in embedded tissue showed a viridions like particles. Also, leukopenia, lymphopenia, al serum increases of CRP and D-dimer, all together suggesting COVID19 disease. But because the vascular pathology observed in this case does not appear in the simple brain abscesses reported. Because it was also positive for ACE2, angiotensin, and COVD19 viral proteins, in an asymptomatic patient for covid19 but with high risk factors such as congenital heart disease, and presenting a cachectic state, a state of immunodeficiency. The co-expression of two or more causative agents of a brain abscess. We should encounter much more patients with BA since the degree of the pandemic. False positives, false negatives and negative patients are increasingly reported.
Author Statements
Footnotes
Declaration of interest: We declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Author Contributions
Conceptualization: MLTS; Data curation: MLTS; Funding acquisition: MLTS; Investigation: MLTS, CSL; Methodology: MLTS; Project administration: MLTS; Resources: MLTS, AOP; Software: MLTS.GC; JLSH; Supervision: MLTS.GC; JLSH, CSL; Validation: MLTS, MCR, AOP, SM, CSL; Visualization: MLTS.GC; JLSH, JL; AOP; Writing – original draft: MLTS.GC; JLSH, JL; Writing – review & editing: MLTS.GC; JLSH, JL.
Conflict of Interest Statement
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Ethics
Where we don’t require, informed consent four each patient procedure.
Funding
We had no funding for this study.
Disclosure
We have no conflict of interest to disclose.
References
- Brouwer MC, van de Beek D. Epidemiology, diagnosis, and treatment of brain abscesses. Curr Opin Infect Dis. 2016; 30: 129-134.
- Asmita Ghosh, Anusua Sarkar, Pubali Paul, Parth Patel. The rise in cases of mucormycosis, candidiasis and aspergillosis amidst COVID19 Fungal Biol Rev. 2021; 38: 67-91.
- Gupta V, Singh P, Sukriti K. Fungal brain abscess in a post COVID-19 patient. BMJ Case Rep. 2021; 14: e246319.
- Cox MJ, Loman N, Bogaert D, O’Grady J. Co-infections: potentially lethal and unexplored in COVID-19. The Lancet Microbe. 2020; 1: e11.
- Lou JJ. Movassaghi M, Gordy D, Olson MG, Zhang T, Khurana MS, et al. Neuropathology of COVID-19 (neuro-COVID): clinicopathological update. Free Neuropathology. 2021; 2: 2-8.
- Mukerji S, Solomon I. What can we learn from brain autopsies in COVID-19? Neuroscience Letters 742 (2021) 135528.
- Maiese A, Manetti AC, Bosetti C, Del Duca F, La Russa, Frati P, et al. SARS-CoV-2 and the brain: A review of the current knowledge on neuropathology in COVID-19. Brain Pathology. 2021; 1: e13013.
- Chen X, Liao B, Cheng L, Peng X, Xu X, Li Y, et al. The microbial coinfection in COVID-19. Appl Microbiol Biotechnol. 2020; 104: 7777–7785.
- Talamonti G, Colistra D, Crisà f, Cenzato M Giorgi P, D’Aliberti A, Spinal epidural abscess in COVID-19 patients. Journal of Neurology. 2021; 268: 2320-2326.
- Solomon IH, Normandin E, Bhattacharyya S, Mukerji SS, Keller K, Ali AS, et al. Neuropathological Features of COVID-19. N Engl J Med. 2020; 383: 989–992.
- Lee MH, Perl DP, Nair G, Li W, Maric D, Murray H, et al. Microvascular Injury in the Brains of Patients with COVID-19. N Engl J Med. 2021; 384: 481-483.
- Justin K. Achua, Kevin Y Chu, Emad Ibrahim, Kajal Khodamoradi, Katiana S Delma, Oleksii A Iakymenko, et al. Histopathology and Ultrastructural Findings of Fatal COVID-19 Infections on Testis. World J Mens Health. 2021; 39: 65–74.
- Wong DW L, Klinkhammer BM, Djudjaj S, Villwock S, Timm MC, Buhl EM, et al. Multisystemic Cellular Tropism of SARS-CoV-2 in Autopsies of COVID-19 Patients. Cells. 2021; 10: 1900.
- Lagunas-Rangel FA. Neutrophil-to-lymphocyte ratio and lymphocyte-to-C-reactive protein ratio in patients with severe coronavirus disease 2019 (COVID-19): a meta-analysis. J Med Virol. 2020; 92: 1733-1734.