
Case Report
Austin J Clin Case Rep. 2024; 11(2): 1318.
Late Nosocomial Purulent Diffuse Peritonitis
Teymurov EZ*; Namazov AE; Bayramov NY
Department of 1st Surgical Diseases, Azerbaijan Medical University, Turkey
*Corresponding author: Teymurov EZ Department of 1st Surgical Diseases, Azerbaijan Medical University, Samad Vurghun, Baku, Nasimi, AZ1022, Azerbaijan, Turkey. Tel: +994 513340859 Email: elchin1997@gmail.com
Received: March 01, 2024 Accepted: April 08, 2024 Published: April 15, 2024
Abstract
A 31-year-old male patient was admitted with complaints of widespread abdominal pain, high temperature (Tmax 39.2°C), lack of appetite, weakness, weight loss. The patient underwent “Laparoscopic fundoplication in Toupet modification” for GERD 10 days ago. The early postoperative period passed without peculiarities. On the 5th postoperative day, the patient had abdominal pain, lack of appetite, weakness. The patient was treated with symptomatic therapy according to the doctor’s recommendations, but without any effect. On the 10th day the patient with the above complaints came to the clinic. On examination “plank-shaped abdomen”, peritoneal symptoms were positive, intestinal peristalsis was sluggish. In laboratory parameters the level of CRP - 339 mg/ml, no changes in clinical blood analysis were detected. The patient underwent diagnostic laparoscopy, the revision revealed diffuse purulent peritonitis, no defects of the walls of the gastrointestinal tract organs were revealed, the place of fundoplication without features, the sutures were sound. The abdominal cavity was drained: right and left lateral canals, subhepatic space and small pelvis. Antibacterial therapy has been assigned: meropenem 1.0 x3 i/v, metronidazole 500 mg x2 i/v, massive infusion therapy and parenteral nutrition. Positive clinical and laboratory dynamics was observed in the postoperative period. On the 9th postoperative day, the level of CRP decreased to 43 mg/ml, the patient was discharged for outpatient treatment in satisfactory condition.
Keywords: Spantaneus peritonitis; Laparoscopy surgery; MRSA; Sepsis
Abbreviations: HR; WBC; Hb; PLT; CRP; ALT; AST; ALP; GGT; MRSA; SBP; UTI; CT; PIAI, AbSeS
Introduction
The article describes a case of idiopathic (spontaneous) purulent peritonitis developed after surgical treatment, describes the tactics of surgical and conservative treatment of this pathology.
Case Report
A 31-year-old male patient came to the clinic with complaints of "widespread abdominal pain, hyperthermia, nausea, lack of appetite, general weakness and fatigue, weight loss". On examination - skin and visible mucous membranes are pale, clean, peripheral lymph nodes are not palpated, no edema. Auscultation in the lungs vesicular breathing, no rales. Heart tones are rhythmic, HR - 118/min. The abdomen on palpation is board-shaped, painful in all parts, Shchotkin-Blumberg and Mendel's symptom is positive. Intestinal peristalsis was weakened. Diuresis is free, oliguria is noted. There's been no defecation since last day. Laboratory parameters at the moment of hospitalisation: WBC- 7.1 x109/l, Hb-13.1 g/dL, PLT- 327 x109/l, CRP - 339 mg/ml, ALT - 23, AST - 41, ALP - 80, GGT - 18, Total bilirubin - 0.6, Direct bilirubin - 0.4, Albumin - 2.8, Amilaza - 39, Kreatinn - 0.9. The patient underwent computer tomography of abdominal cavity organs and diffuse peritonitis was confirmed. The patient underwent diagnostic laparoscopy (Figure 1, Figure 2). At revision the abdominal cavity was diffusely covered with purulent contents, the consistency of the fundoplication site, integrity of the stomach, small and large intestine walls was assessed - no defects were revealed (Figure 3, Figure 4). During the intervention, 100 ml of methylene blue solution was administered through a nasogastric tube. When assessing the anastomosis integrity, no extravasation was detected, the integrity of the anastomosis was preserved. Purulent film was taken for bacterial culture. Sanation of the abdominal cavity was performed. Drainage of the right and left lateral canal, subhepatic space and small pelvis was performed. Antibacterial therapy was started for the patient in the postoperative period - meropenem 1.0 x3 i/v, metronidazole 5%-100.0 i/v, massive infusion therapy at the rate of 30 ml/kg/day, parenteral nutritional support was started. On the 1st postoperative day positive clinical dynamics was observed: abdominal pain decreased, diuresis normalized, the patient became active, the volume of infusion therapy was reduced to 20 ml/kg/day. Serous-purulent content with a total volume of 130 ml was noted from the drains. On the 2nd day CRP level decreased to 298 mg/ml. On the 24th day no contents were released from the left lateral canal drain, the drain was removed. On the 3rd day parenteral nutrition was suspended, enteral nutrition was administered, Srb level decreased to 139 mg/ml. The drains from the pelvis and right lateral canal were removed. On the 4th postoperative day, there was no discharge from the drain installed in the subhepatic space, the drain was removed. According to the results of bacteriological analysis of purulent abdominal cavity contents, MRSA and Streptococcus anginosus were isolated, sensitive to meropenem, linezolid and ceftazidime with sulbactam. On the 9th postoperative day, the patient underwent control abdominal CT - pathological changes wasn’t detected and he was discharged for outpatient treatment in satisfactory condition.
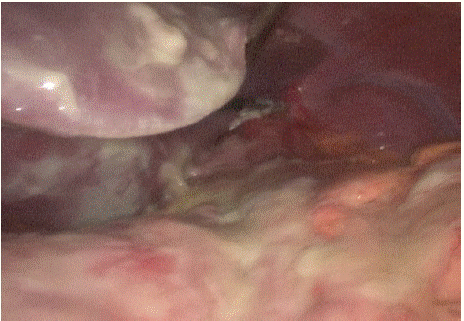
Figure 1: Subhepatic area.
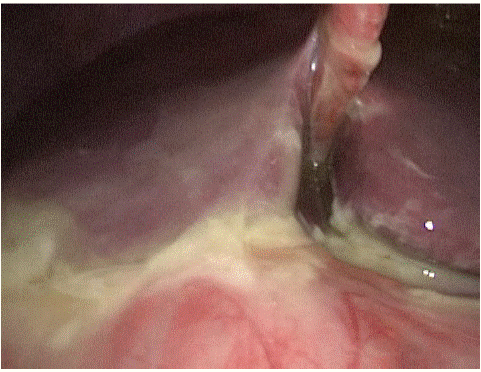
Figure 2: Purulent diffuse peritonitis.
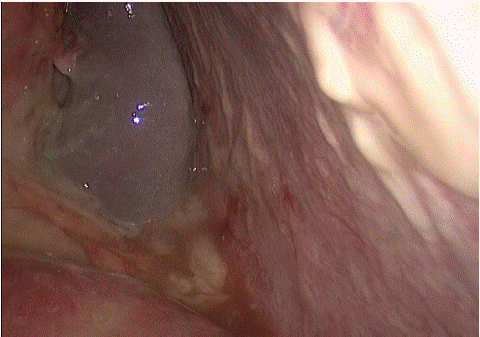
Figure 3: Left abdomenal canal.
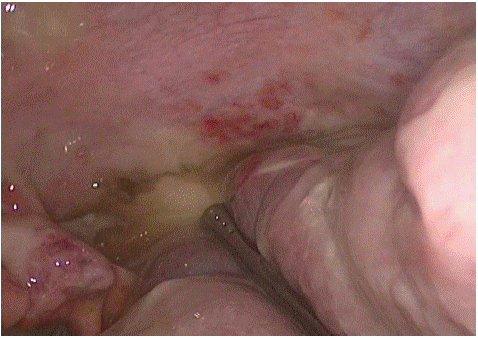
Figure 4: Pelvic cavity.
Discussion
Peritonitis is a life-threatening condition characterized by high mortality in surgical patients. According to the latest classification, peritonitis with no apparent source of origin is commonly referred to as primary or spontaneous peritonitis. At present, this pathology can be difficult to diagnose due to the absence of hyperthermia and leukocytosis [1]. Most often spontaneous peritonitis develops in the presence of ascitic fluid in the abdominal cavity. Bacterial infections are common in patients with cirrhosis and are a necessary reason for hospitalization of these patients. Infections may occur on admission or develop during hospitalization in 25-35% of cases, with an incidence 4-5 times higher than in the general population [2]. The most common infections are Spontaneous Bacterial Peritonitis (SBP 27%), followed by urinary tract infections (UTI 22%) and pneumonia (19%). A higher prevalence of pneumonia and UTIs and a lower prevalence of SBP were found in Asian centres compared with centres in the Americas and Europe [3]. Pathogenesis involves bacterial translocation, probably via specialized epithelial M cells located in the intestinal peyer's plaques covering specialized subepithelial mesenteric lymphatic tissue, with the bacteria entering the lymphatic circulation and eventually the bloodstream in the context of compromised host defence and impaired immune function [4]. In most cases, MRSA accounts for at least 25-50% of S. aureus infections in hospitals [5]. MRSA-induced peritonitis has been described in the literature in association with haematogenous spread of bacteria from infected permanent catheter, nasal carriers, skin lesions or in the setting of peritoneal dialysis [6]. In our opinion, the development of peritonitis in the patient resulted from a hospital-acquired intra-abdominal infection. MRSA bacteria entered the abdominal cavity as a result of surgical treatment. Given the peculiarities of these bacterial strains, the use of antibiotic prophylaxis and subsequent antibiotic therapy was of no clinical significance in suppressing the development of this microorganism [7]. According to the "AbSeS" classification based on the detection of methicillin-resistant Staphylococcus aureus cultures allows judgement of late nosocomial infection [8]. According to their data, the mortality rate in patients with spontaneous peritonitis is about 29%. The recommended antibiotic therapy in this case is carbopenems or oxazalidinones. Empirical antibiotic therapy when the diagnosis of spontaneous generalized peritonitis is confirmed increases the overall survival of patients [9].
As for repeated surgical interventions, Bacteraemia caused by intra-abdominal surgical infection is not uncommon, its incidence is 10-26% in repeated operations [10,11] and increases in case of late diagnosis [12], septic shock [10] or multidrug resistance [13].
In cases of delayed diagnosis [12], septic shock [10] or multidrug resistance [13], this rate increases. Patients who develop Postoperative Intra-Abdominal Infection (PIAI) have an increased risk of unfavourable outcome and mortality. Interestingly, the literature lacks specific data on bacteraemia during PIAI. However, only 39% of patients had secondary peritonitis, and the proportion of PIAI cases was not specified [14].
References
- Antonio Facciorusso, Matteo Antonino, Eugenio Orsitto, Rodolfo Sacco «Primary and secondary prophylaxis of spontaneous bacterial peritonitis: current state of the art» Expert Rev Gastroenterol Hepatol. 2019; 13:751-759.
- Spontaneous bacterial peritonitis: update on diagnosis and treatment Roxana-Emanuela Popoiag 1, Carmen Fierbinteanu-Braticevici Rom J Intern Med. 2021; 59: 345-350.
- Piano S, Singh V, Caraceni P, Msiwall R, Alessandria C, Fernanfez J, et al. Epidemiology and effects ofbacterial infections in patients with cirrhosis worldwide. Gastroenterology. 2019; 156: 1368–1380.
- Biggins SW, Angeli P, Garcia-Tsao G et al. Diagnosis, evaluation, and management of ascites, spontaneous bacterial peritonitis and hepatorenal syndrome: 2021 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology. 2021; 74: 1014–1048.
- Diekema DJ, Pfaller MA, Schmitz FJ, Smayevsky J, Bell J, Jones RN, et al. Survey of infections due to Staphylococcus species: frequency of occurrence and antimicrobial susceptibility of isolates collected in the United States, Canada, Latin America, Europe, and the Western Pacific region for the SENTRY Antimicrobial Surveillance Program, 1997-1999. Clin Infect Dis. 2001; 32: S114–S132.
- Salzer WL: Peritoneal dialysis-related peritonitis: challenges and solutions. Int J Nephrol Renovasc Dis. 2018; 11: 173-86.
- Matteo Bassetti, Christian Eckmann, Daniele Roberto Giacobbe, Massimo Sartelli, Philippe Montravers «Post-operative abdominal infections: epidemiology, operational definitions, and outcomes» Intensive Care Med. 2020; 46: 163-172.
- Stijn Blot, Massimo Antonelli, Kostoula Arvaniti, Koen Blot, Ben Creagh-Brown, Dylan de Lange, et al. Epidemiology of intra-abdominal infection and sepsis in critically ill patients: “AbSeS”, a multinational observational cohort study and ESICM Trials Group Project. Intensive Care Med. 2019; 45: 1703-1717.
- Philip Kam-Tao Li, Kai Ming Chow, Yeoungjee Cho, Stanley Fan, Ana E Figueiredo, Tess Harris, et al. ISPD peritonitis guideline recommendations: 2022 update on prevention and treatment. Perit Dial Int. 2022; 42: 110-153.
- Riche FC, Dray X, Laisne MJ, Mateo J, Raskine L, Sanson-Le Pors MJ, et al. Factors associated with septic shock and mortality in generalized peritonitis: comparison between community-acquired and postoperative peritonitis. Crit Care. 2009; 13: R99.
- Montravers P, Augustin P, Grall N, Desmard M, Allou N, Marmuse JP, et al. Characteristics and outcomes of anti-infective de-escalation during health care-associated intra-abdominal infections. Crit Care. 2016; 20: 83.
- Roehrborn A, Thomas L, Potreck O, Ebener C, Ohmann C, Goretzki PE, et al. The microbiology of postoperative peritonitis. Clin Infect Dis. 2001; 33: 1513–9.
- Seguin P, Fedun Y, Laviolle B, Nesseler N, Donnio PY, Malledant Y. Risk factors for multidrug-resistant bacteria in patients with post-operative peritonitis requiring intensive care. J Antimicrob Chemother. 2010; 65: 342–6.
- Adel Alqarni, Elie Kantor, Nathalie Grall, Sebastien Tanaka, Nathalie Zappella, Mathieu Godement, et al. Clinical characteristics and prognosis of bacteraemia during postoperative intraabdominal infections. Critical Care. 2018; 22: 175.