
Case Report
Austin J Clin Case Rep. 2024; 11(3): 1322.
A Case of Disseminated Coccidioidomycosis with Aseptic Meningitis and Erythema Nodosum in an Immunocompetent Host
Sood Nikhil, MD1*; Meyer Danielle2; Oommen Jacob, MD3; Parlapalli Roop, MD, FACP, FHM4
1Department of Medicine, Banner Gateway Medical Center, Gilbert, AZ, USA
2OMS-4, ATSU-KCOM, USA
3Synergy Infectious Disease, Gilbert, AZ, USA
4Department of Medicine, Geisinger Community Medical Center, Scranton, PA, USA
*Corresponding author: Sood Ni MD Department of Medicine, Banner Gateway Medical Center, 1900 N Higley Rd. Gilbert, AZ, 85234 USA. Tel: 480-543-2034; Fax: 480-543-2647 Email: nikhil.sood@bannerhealth.com
Received: May 08, 2024 Accepted: June 03, 2024 Published: June 10, 2024
Abstract
A 55-year-old immunocompetent female presented with shortness of breath for six weeks, ongoing headaches, and localized redness of the left foot that started three days prior to presentation. The workup revealed multiple small pulmonary nodules bilaterally. Serum cocci EIA IgM was positive, indicating acute infection. Due to persistent headaches, a lumbar puncture was performed, which confirmed aseptic meningitis. Furthermore, the patient developed multiple painful, erythematous subcutaneous nodules during the hospital stay. A month ago, our patient presented with shortness of breath symptoms and was treated for community-acquired pneumonia. She developed a rash that was labeled as a penicillin allergy-related rash. During this admission, she was treated for cellulitis, this time initially when she had erythema nodosum on presentation. The novelty of this case lies in its rarity and the diagnostic dilemma it posed, ultimately leading to successful management through accurate diagnosis. She was initiated on fluconazole 800 mg daily and was doing well at a two-week follow-up. Disseminated coccidioidomycosis with meningitis and erythema nodosum in immunocompetent patients is rare and is associated with significant morbidity and mortality. Even with complete treatment, the rate of relapse is high, necessitating regular follow-up and monitoring of complement fixation titers.
Keywords: Disseminated coccidioidomycosis; Erythema nodosum; Aseptic meningitis
Background
Coccidioidomycosis, also known as Valley fever, is a fungal infection caused by inhaling spores of the Coccidioides species. Most infections are endemic to the Southwest United States (including Arizona and California), Mexico, and Latin America [1]. The incidence has dramatically increased over the past two decades. Reports show that 150,000 new infections occur annually. Less than 1% of cases are disseminated, of which one-third are fatal [2]. Disseminated disease is still uncommon and primarily reported in immunocompromised individuals. We report a case of disseminated coccidioidomycosis in an immunocompetent patient.
Case Presentation
Our patient is a 55-year-old female who initially presented for localized redness of the left foot that started three days prior to the presentation. Medical history was significant only for mitral valve prolapse. She had been residing in Arizona for over a decade. She also reported ongoing fevers and cough for six weeks. Three weeks before the current presentation, she was hospitalized for community-acquired pneumonia. At that time, Computed Tomography (CT) chest revealed a left lower lobe consolidation and an incidental 5 mm right upper lobe pulmonary nodule. Viral multiplex respiratory panel and cocci serology were negative. The patient initially received ceftriaxone 2 g IV daily and azithromycin 500 mg IV daily but developed an erythematous macular rash on her chest and back about 48 hours after drug administration. Beta-lactam allergy was suspected, and antibiotics were switched to levofloxacin 750 mg IV q24h. After 24 hours of monitoring the rash, she was discharged home on a 7-day course of oral cefdinir 300 mg twice daily and azithromycin 500 mg daily. She reported her cough improved, but fevers recurred intermittently, and she felt fatigued.
In the emergency department, she had a fever of 38.2 C and a heart rate of 102 bpm. Physical examination demonstrated localized erythema over the dorsomedial aspect of the left foot and left the popliteal region with associated warmth but no edema or tenderness. Labs revealed an elevated WBC count (16.2 k/μL), elevated ANC (11.4 k/μL), and normal lactic acid (1.3 mmol/L). The respiratory viral panel was negative. Chest x-ray showed no acute findings. The lung sounds were clear, and she didn’t need supplemental oxygen. Given the concern for sepsis secondary to left lower extremity cellulitis, she was admitted and initiated on vancomycin 1,250 mg IV daily and clindamycin 600 mg IV q8h. Approximately 24 hours later, the patient developed painful, erythematous subcutaneous nodules scattered across all four extremities, diffuse arthralgias, headache, and night sweats. At this point, there was a concern for coccidioidomycosis. Fluconazole 400 mg oral daily was started.
CT chest revealed the prior left lower lobe consolidation had decreased in size. However, there were numerous bilateral pulmonary nodules, with a lower lobe predominance. The right upper lobe nodule resolved. Serum cocci EIA IgM was positive, indicating acute infection. Serum cocci EIA IgG and serum cocci complement fixation (<1:2) were negative. Upon further history, the patient mentioned off-and-on headaches for the last few weeks. These were atypical for her as she had no known migraine or headache history. CT head was negative. Due to the headaches, fluconazole was increased to 800 mg daily, and a lumbar puncture was performed. CSF fluid analysis revealed elevated WBC count (40 /μL) with 95% lymphocyte predominance and normal levels of glucose (63 mg/dL) and protein (31.6 mg/dL). CSF complement fixation was negative. CSF culture showed no growth, indicating aseptic meningitis. The final diagnosis was thus disseminated coccidioidomycosis with meningitis and erythema nodosum. Four days after starting fluconazole, the skin lesions began to recede, and fevers slowly started trending down. She was discharged on fluconazole and was doing well at a two-week follow-up.
Discussion
This case report describes a rare case of disseminated coccidioidomycosis in an immunocompetent patient involving the skin and nervous system in an individual with no risk factors related to spore exposure, such as farming. Coccidioidomycosis is a systemic fungal infection caused by Coccidioides immitis or Coccidioides posadasii. It is a significant fungal disease found in many desert regions of the Western Hemisphere. The inhaled organisms are highly pathogenic, but only half of infected, immunologically intact people develop symptomatic pneumonia; most symptomatic infections resolve spontaneously, although some resolve very slowly. Furthermore, second infections are sporadic, and natural immunity after infection is robust [3]. Primary pulmonary disease is the most common initial source of infection, with associated low-grade fevers, headaches, and body aches. Reported cases of Coccidioides infections have been increasing for several decades to about 20,000 in 2019, with most cases being in the Arizona and California regions. There are approximately 200 yearly Coccidioidomycosis-associated deaths in the United States from the years 1999–2019 [4]. The diagnostic approach starts with serological testing in suspected cases. Due to increased sensitivity, Enzyme-linked Immunoassays (EIA) for IgM/IgG are the most common initial evaluation step. They are followed up by an immunodiffusion assay for quantitative analysis to monitor response to therapy [5].
Progression to disseminated disease occurs in about one percent of cases. It occurs in 30%–50% of immunosuppressed hosts and can be single-site or multisite [2]. It often develops through the hematogenous or lymphatic spread. It can involve any organ, but the involvement of the skin, central nervous system, and musculoskeletal system are reported to be most prevalent [6]. Risk factors for dissemination include various immunocompromised states such as HIV as well as reported genetic defects involving interleukin-12/interferon-gamma pathways, which are more common in African American, Filipino, and Hispanic populations [2].
Hypersensitivity reactions, such as erythema multiforme and erythema nodosum, are common cutaneous presentations that can co-occur during an acute primary pulmonary infection [7]. Populations at increased risk primarily work outdoors or live in an endemic area. Additional risk factors include immunosuppressive medications, diabetes mellitus, and pregnancy [1]. 30% of patients can develop tender erythema nodosum lesions over the shins several weeks after pulmonary symptoms resolve. Cutaneous manifestations are rare and are a sign of a more severe infection with poorer outcomes [8]. Our patient was initially treated for cellulitis when she had erythema nodosum on presentation.
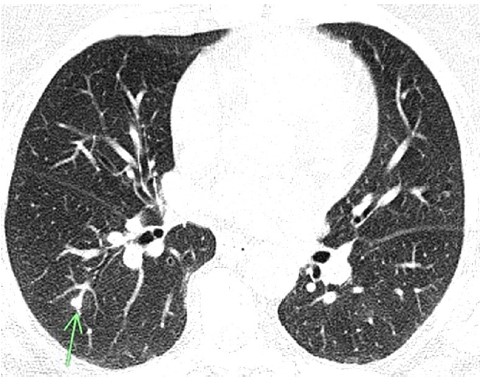
Figure 1: CT Chest film showing a nodule seen in coccidioidomycosis.
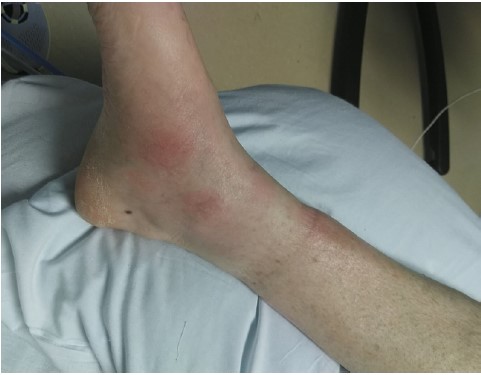
Figure 2: Erythema Nodosum on the leg.
Meningitis is the most devastating complication of coccidioidal infections. The most common presentation is headache. A high index of suspicion and early lumbar puncture, either with or without antecedent neuroimaging, is paramount [9]. Per the CDC, a thorough workup includes serologic testing for IgM and IgG antibodies against Coccidioides and obtaining cultures from blood, sputum, CSF, and urine [10]. A 2023 systematic review of available literature examined meningeal coccidioidomycosis. Four of the fourteen eligible cases were females (28.57%), with the remaining ten being males (71.42%). Thirteen were in HIV-negative individuals (92.8%), while only one was in HIV-positive individuals (7.14%). The recovery rate out of these 14 individuals was 10 (71.42%), while three expired (21.4%), and one was comatose (7.14%). CSF cocci titers were reported high in about seven cases [11]. A positive CSF complement fixation (CF) titer at a reliable laboratory is also helpful but frequently negative. A positive serology (blood) with ID IgG or CF antibody is the most common way the diagnosis is made [12].
Recently, a small number of patients have been identified with specific gene mutations that alter immunologic responses involving interferon-γ, interleukin 12 (IL-12), and other cellular immune pathways that appear to be responsible for their progressive coccidioidal infections. In such patients, the risk of disseminated infection can be as high as 75% [13]. The duration of treatment varies based on the resolution of symptoms and stable improvement of titers. Specifically, for disseminated coccidioidomycosis, treatment is recommended for a minimum of 3 years with stable imaging results and clinical status and a complement fixation titer of less than 1:2 for at least six months. Untreated or incomplete treatment can cause mortality [14].
Spinal Fluid
Patient value
Reference Range
Glucose, CSF
63 mg/dL
40 - 70 mg/dL
Protein, CSF
32 mg/dL
15 - 45 mg/dL
RBC, CSF
<2 /uL
0 μL
WBC, CSF
40 μL
0 μL
Lymphocytes, CSF
95%
40-80%
Coccidioides, CSF
<1:1
< 1:1
Table 1: CSF findings.
Our patient was not diabetic, not pregnant, and had no history of immunocompromising states. She had no previous history of allergies and was not directly exposed to soil (occupational history). Her HIV and CD4 counts were normal. We couldn’t identify any risk factors from the list above, which makes it quite interesting. Furthermore, aseptic meningitis is also not that common. The spinal fluid culture and Coccidioides Comp Fix were reported to be less than 1:2. Usually, titers >1:16 are associated with disseminated disease. Only serum Coccidioides EIA IgM Ab was positive in our patient. As discussed above, the CSF cocci titers can be negative. Also, the CSF and serum test can be initially falsely negative in acute infections. Our patient was doing well at a two-week follow-up. Her headache, low-grade fevers, fatigue, and tiredness improved. The novelty of this case lies in its rarity and the diagnostic dilemma it posed, ultimately leading to successful management through accurate diagnosis.
Conclusion
Disseminated coccidioidomycosis is uncommon in immunocompetent patients. We highlight the importance of disseminated coccidioidomycosis and its appropriate workup in an immunocompromised patient. Complement fixation titers during active therapy, as increasing titers suggest active disease.
Author Statements
Conflict of Interest
The authors declare no conflict of interest.
References
- Brown J, Benedict K, Park BJ, Thompson GR. Coccidioidomycosis: epidemiology.Clin Epidemiol. 2013; 5: 185–197.
- Odio CD, Marciano BE, Galgiani JN, Holland SM. Risk Factors for Disseminated Coccidioidomycosis, United States. Emerg Infect Dis. 2017; 23: 311.
- Kirkland TN, Hung C-Y, Shubitz LF, Beyhan S, Fierer J. The Host Response to Coccidioidomycosis. Journal of Fungi. 2024; 10: 173.
- Valley Fever statistics | coccidioidomycosis | types of fungal diseases | fungal | CDC. 2022.
- Johnson RH, Sharma R, Kuran R, Fong I, Heidari A. Coccidioidomycosis: A Review. Journal of Investigative Medicine. 2021; 69: 316-323.
- Desai SA, Minai OA, Gordon SM, O’Neil B, Wiedemann HP, Arroliga AC. Coccidioidomycosis in non-endemic areas: a case series. Respir Med. 2001; 95: 305-9.
- Garcia Garcia SC, Salas Alanis JC, Flores MG, Gonzalez SE, Cabrera LV, Candiani JO. Coccidioidomycosis and the skin: a comprehensive review. An Bras Dermatol. 2015; 90: 610–619.
- Brown M, Zhou AE, Jaffe DF, Pfau RG. Cutaneous Coccidiomycosis. Am J Dermatopathol. 2024; 46: 111-113.
- Johnson RH, Sharma R, Kuran R, Fong I, Heidari A. Coccidioidomycosis: A Review. Journal of Investigative Medicine. 2021; 69: 316-323.
- Centers for Disease Control and Prevention. Information for healthcare professionals about valley fever (Coccidiomycosis). 2021. Centers for Disease Control and Prevention.
- Ghantarchyan H, Oganesian B, Gayed MM, Maknouni B, Hasan M. A Rare Case of Coccidioidomycosis Meningitis. J Med Cases. 2023; 14: 81-87.
- Christelle Kassis, Zaidi S, Kuberski T, Moran A, Gonzalez O, Hussain S, et al. Role of Coccidioides Antigen Testing in the Cerebrospinal Fluid for the Diagnosis of Coccidioidal Meningitis, Clinical Infectious Diseases. 2015; 61: 1521–1526.
- Galgiani JN, Ampel NM, Blair JE, Catanzaro A, Geertsma F, Hoover SE, et al. 2016 Infectious Diseases Society of America (IDSA) Clinical Practice Guideline for the Treatment of Coccidioidomycosis, Clinical Infectious Diseases. 2016; 63: e112–e146.
- Amina Jamali, Huy Nguyen, Patricia Tran, Cheng Chen, Timothey Devine, Saba Ahmad. A Rare Case of Disseminated Coccidioidomycosis Presenting as an Intracardiac Mass. AIM Clinical Cases. 2024; 3: e220859.