
Case Report
Austin J Clin Case Rep. 2024; 11(7): 1343.
Perioperative Aneurysmal Subarachnoid Hemorrhage During Acute Aortic Endocarditis Heart Surgery
Uhl E1; Voigtmann H2*; Schneck E3; Rutsatz W4; Struffert T1; Haj M5; Roth P5; Bender M2
1Department of Neuroradiology Justus-Liebig-University Gießen, Gießen, Germany
2Department of Neurosurgery, Justus-Liebig-University Gießen, Gießen, Germany
3Department of Anaesthesiology Justus-Liebig-University Gießen, Gießen, Germany
4Department of Cardiology Justus-Liebig-University Gießen, Gießen, Germany
5Department of Cardiac Surgery Justus-Liebig-University Gießen, Gießen, Germany
*Corresponding author: Hans Voigtmann, Department of Neurosurgery, Justus-Liebig-University Gießen, Klinikstraße 33, 35392 Gießen, Germany. Tel: +49(0)641-98557160; Fax: +49(0)641-98557169 Email: hans.voigtmann@chiru.med.uni-giessen.de
Received: October 26, 2024; Accepted: November 14, 2024 Published: November 21, 2024
Abstract
Backround: Aneurysmal Subarachnoid Hemorrhage (SAH) associated with Infectious Endocarditis (IE) is a rare disease and only a few cases have been reported in literature, posing a challenge to Intensive Care Unit (ICU) treatment with opposing therapeutic hemodynamic strategies.
Case presentation: A 56-year-old patient suffered a general seizure caused by a SAH with subdural and intracerebral hematoma due to a Medial Cerebral Artery (MCA) aneurysm right before open heart surgery for treatment of IE. The heart surgery was aborted, and the aneurysm was treated via coil embolization and the subdural and intracerebral hematoma were removed via craniotomy, following a conservative treatment strategy of the severe aortic regurgitation. The conservative treatment concept of IE was followed until the high-risk phase of vasospasm had passed and 25 days after SAH a bioprosthetic of the aortic valve was implanted. The patient was discharged to a rehabilitation center and survived without any neurological deficit.
Conclusions: This case shows how life-saving a constant reevaluation of the patient’s condition in a multidisciplinary team can be and emphasizes the importance of close communication and its possible success at the edge of modern intensive care medicine.
Keywords: Subarachnoid hemorrhage; Infectious endocarditis; Vasospasm; Interdisciplinary communication; Hemodynamic management
Introduction
Only a few cases of aneurysmal Subarachnoid Hemorrhage (SAH) associated with Infectious Endocarditis (IE) have been reported of which most describe mycotic intracranial aneurysms responsible for parainfectious SAH [2,5]. We present a case of a 56 year old patient, who developed a general seizure and an acute SAH with subdural hematoma in the operating room immediately prior to the induction of general anesthesia for urgent surgical IE treatment. In the following, the heart surgery was aborted, the aneurysm secured via coil embolization, and the subdural hematoma surgically evacuated via craniotomy. Even though SAH in IE displays a rare condition, both diseases pose a challenge to Intensive Care Unit (ICU) treatment with opposing therapeutic hemodynamic strategies. This case shows the life-saving effect of a constant re-evaluation of the patient’s condition in a multidisciplinary team approach and emphasizes the importance of close communication.
Case Presentation
A 56-year-old patient presented with a reduced general condition, shortness of breath, and acute kidney failure. Transesophageal echocardiography showed an acute endocarditis of the aortic valve, which originated from a septic bloodstream infection with streptococcus mutans. Perforation of the non-coronary cusp of the aortic valve caused a severe aortic regurgitation with dilatation of the left ventricle. Consequently, surgical treatment was indicated by a high-grade aortic valve regurgitation (Figure 1) and menacing left ventricular decompensation.
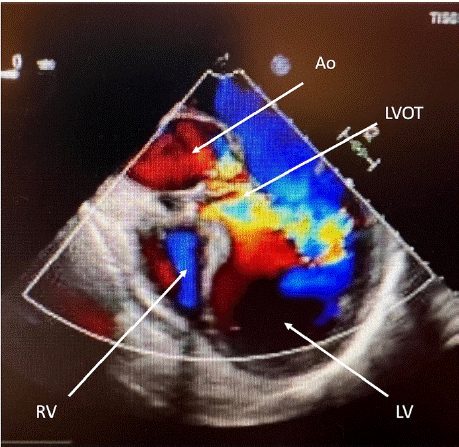
Figure 1: Transesophageal echocardiography: left Ventricle (LV), Right
Ventricle (RV), ascending aorta (AAo) and left ventricular outflow track (LVOT)
showing high grade aortic regurgitation due to aortic valve endocarditis with a
pressure halftime (PHT) of 180ms.
In the operating room, immediately before the induction of general anesthesia the patient developed a general seizure. Due to the loss of consciousness, the patient was intubated and mechanically ventilated. The urgent heart operation was cancelled, and a Cranial Computed Tomography (CCT) was performed which showed a severe subarachnoid hemorrhage, characterized as Fisher grade IV, a subdural hematoma and Intracerebral Hemorrhage (ICH) (Figure 2). In the cerebral panangiography an aneurysm of the right Middle Cerebral Artery (MCA) was identified.
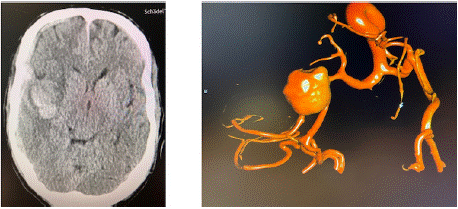
Figure 2: CCT with acute subarachnoid hemorrhage, subdural hematoma
and intracerebral hemorrhage (left picture), of a ruptured aneurysm of the
right medial cerebral artery in digital subtraction angiography (right picture).
Due the severe cardiac failure, an interdisciplinary consent for an endovascular treatment of the aneurysm followed by surgical evacuation of the intracerebral and subdural hematoma was determined. Thus, immediate coil embolization of the aneurysm was performed, followed by craniotomy and hematoma evacuation. After surgical treatment the patient was transferred to our neurosurgical ICU, were an external ventricular drainage was implanted. The patient was sedated and mechanically ventilated with stable vital signs and low catecholamine requirements. Postoperative computed tomography scans of the skull showed a regular postoperative finding (Figure 3).
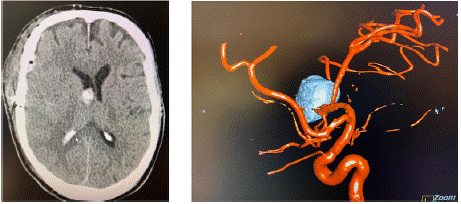
Figure 3: Postoperative CCT (left image) and angiography (right image)
show evacuation of the subdural and intracerebral hematoma and the
occlusion of the aneurysm by coils.
In accordance with the microbiological resistance testing the patient's antibiotic therapy was continued with ceftriaxone 2x 2g/d for five weeks for treatment of the aortic valve endocarditis. Despite the untreated severe aortic valve insufficiency, the patient remained hemodynamically stable with low catecholamine requirements (1-8 μg/min). Differentiated volume- and catecholamine therapy was administered with a target of systolic blood pressure of 140- 160 mmHg using noradrenaline and low doses of dobutamine (80 – 170μg/min). Furthermore, the calcium antagonist nimodipine (2mg/h) was given for prevention of vasospasm. This treatment was performed with regular bedside echocardiographic follow-ups of the left ventricle in the event of impending decompensation and severe aortic valve regurgitation especially with elevated cardiac afterload for the treatment of cerebral vasospasm and optimal cerebral perfusion. Furthermore, daily transcranial Doppler examinations for detection of cerebral vasospasm was performed.
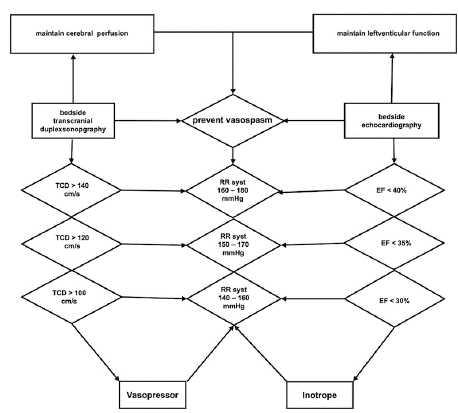
Figure 4: Systematic overview of the ICU management of systolic blood
pressure (RR syst) to prevent cerebral vasospasm under frequent bedside
transcranial duplex sonography (TCD) and control of left ventricular
decompensation by frequent bedside echocardiography under measurement
of the ejection fraction (EF).
Due to the ongoing renal failure requiring dialysis, Intermittent Veno-Venous Hemodialysis (IVVH) was performed throughout the entire hospital stay. Initially, there was a suspicion of an autoimmuneinduced renal insufficiency. Therefore, in close consultation with the colleagues of the nephrology department, a temporary immunosuppressive therapy with prednisolone was administered.
When the laboratory inflammation parameters increased and infiltrates were detected on the chest X-ray on the 5th postoperative day, an empirical antibiotic therapy with meropenem was administered. Under this treatment, the inflammatory parameters significantly declined, and the antibiotic therapy with meropenem could be discontinued. During the treatment on the intensive care unit an extensive diagnostic search for the infectious focus was performed. Also, in the context of a dental consultation due to the initial finding of streptococcus mutans in blood cultures, no infection focus could be detected.
With expected long-term ventilation, primary percutaneous dilatation tracheotomy was performed. After that, the analgesic and sedative medications could be reduced. On the 10th postoperative day, the patient opened his eyes and followed simple instructions, however, the patient showed a hemiparesis with a strength of 3/5 in his left arm and leg.
The conservative treatment concept of IE was followed until the high-risk phase of vasospasm had passed and the external ventricular drainage, which had been placed initially because of a hydrocephalus, could be removed. Finally, 25 days after the SAH, open heart surgery was performed in a risk benefit assessment with a stable clinical condition and CCT scans showing no signs of rebleeding.
A 29mm Hancock bioprosthetic of the aortic valve was implanted, and due to an annular abscess, a patch plastic was performed. In addition, a Left Internal Mammary Artery (LIMA) to the marginal branch bypass was performed in the presence of 1 vessel Coronary Artery Disease (CAD). In the postoperative transesophageal and later transthoracic echocardiograms, the bioprosthetic valve was seen to be in good function (Table 1). The patient was awake and responsive a few hours after the heart surgery without a new neurological deficit.
Preoperative
Postoperative
LVEDD
62mm
45mm
LVESD
52mm
32mm
VST
11
13
PWT
13
13
LVEF
45%
59%
Vena contracta
7,2mm
-
PHT
180ms
-
Table 1: Pre- and postoperative echocardiographic parameters: LVEDD: Left ventricular end-diastolic dimension; VST: Ventricular septal thickness; PWT: Posterior wall thickness; LVMI: Left ventricular mass indexed to body surface area; LVEF: Left ventricular ejection fraction, PHT: pressure halftime. Echocardiography indexes before and after treatment:
The anti-infective therapy with ceftriaxone was continued until the heart surgery. The microbiological control examinations showed no growth of germs in the control blood cultures or in the intraoperative resection of the heart valve. The patient was weaned off the ventilator and decannulated at day 11 after open heart surgery. He suffered from a severe postoperative delirium and due to the ongoing renal insufficiency with insufficient residual urine output, a Quinton catheter was placed without complications and the patient transferred to a rehabilitation center.
Meanwhile, 6 months after discharge from the intensive care unit the patient has fully recovered without focal neurological deficit and is working again. Dialysis is not required anymore.
Discussion
Subarachnoid Hemorrhage and Infectious Endocarditis – A Rare Condition
Aneurysmal SAH remains a devastating disease despite medical advances over the past three decades, with a mortality rate up to 45% within 30 days [4]. Furthermore, over 35% of the surviving patients suffer from a loss of their quality of life with persisting neurological deficit [16].
SAH due to a mycotic aneurysm is a rare complication of IE, with only a small number of cases reported in the literature. Intracranial infectious aneurysms (IIAs), previously referred to as “mycotic aneurysms”, are a rare entity comprising approximately 0.7–5.4% of all cerebral aneurysms [14]. These aneurysms occur in 1–10% of patients with IE [7]. In a data analysis of Singla et al. reported about a lower rate of mortality in patients which were treated by clipping or coiling in comparison to patients receiving conservative treatment. Nevertheless, most of patients within SAH originating from IIAs were treated conservatively [14].
The primary aims of SAH treatment is to stabilize systolic blood pressure, to treat an acute hydrocephalus and to occlude the aneurysm to avoid a fatal rebleeding. The method of aneurysm occlusion depends on the morphology and the location of the aneurysm, the presence of intraparenchymal hemorrhage and the patient’s age. The decision is to be made after a multidisciplinary evaluation between the neurointensivists and endovascular and neurosurgical specialists [10]. Several studies demonstrate no significant long-term advantages of surgical vs. interventional treatment [6,15].
In our case the MCA aneurysm was treated by coil embolization and the subdural and intracerebral hemorrhage were evacuated via craniotomy. In addition, an external ventricular drainage was placed for the treatment of the hydrocephalus.
Subarachnoid Hemorrhage and Infectious Endocarditis – Management of Vasospasm Under Left Ventricular Decompensation
Vasospasm is a common complication of SAH and can lead to significant morbidity and mortality [4]. Management of vasospasm in the Intensive Care Unit (ICU) requires a multimodal approach including close monitoring of blood pressure, use of vasodilators, and other interventions as needed [12]. In general, targeting vasospasm is complicated and only few successful randomized controlled trials in management of vasospasm in aSAH have been published [12].
One of the key elements is the use of vasodilators. The most used vasodilator is nimodipine, a calcium channel blocker, that has been shown to be effective in reducing the incidence and severity of vasospasm in several clinical trials [1,13]. In addition to vasodilators, close monitoring and elevating blood pressure is also important in the management of vasospasm. This includes invasive measurement of blood pressure, and the use of vasopressors to maintain an appropriate cerebral perfusion pressure [12].
Both, SAH and IE pose a huge challenge to ICU treatment with opposing therapeutic strategies. Whereas in treatment of vasospasm after subarachnoid hemorrhage, hypertension and permissive application of crystalloid fluid are indicated and recommended, it is rather obsolete in acute left ventricular decompensation due to aortic regurgitation [11,12]. Heart failure and ventricular decompensation is present in up to 60% of native valve endocarditis and is more often present when IE affects the aortic valve [8]. Usually early surgery is indicated in patients with IE who present valve dysfunction resulting in acute heart failure [11]. However, due to the necessity of anticoagulation and heparinization for performing open heart surgery this would have been associated with a high risk of intracerebral rebleeding for this patient after SAH. Therefore, a conservative therapy of the IE was performed under extended hemodynamic monitoring, repeated echocardiographic examination of the left ventricle, hemodialysis, antibiotic therapy, and repeating interdisciplinary reevaluation.
The patient remained stable under the conservative therapy regime. He showed no signs of left ventricular decompensation under elevated systolic blood pressure 140 – 160 mmHg for vasospasm prophylaxis after a slight increase of TCD value up to 107 cm/s over the right medial cerebral artery on day 5 after SAH. Ejection Fraction (EF) remained decreased but stable with about 45%, the hypertensive therapy was continued until day 14 after SAH and TCD values decreased continuously, and the patient showed a stable neurological condition. The ventricular drainage could be removed after the CCT scans showed stable postoperative findings with no signs of hydrocephalus or new intracranial rebleeding. Thus, after the high-risk phase of postoperative vasospasm was over [3], successful open-heart surgery was performed for treatment of infectious endocarditis after interdisciplinary consultation between neuro- and cardiovascular surgical department 25 days after SAH.
References
- Allen GS, Ahn HS, Preziosi TJ, Battye R, Boone SC, Boone SC, et al. Cerebral arterial spasm--a controlled trial of nimodipine in patients with subarachnoid hemorrhage. N Engl J Med. 1983; 308: 619-624.
- Boukobza M, Duval X, Laissy JP. Mycotic intracranial aneurysms rupture presenting as pure acute subdural hematoma in infectious endocarditis. Report of 2 cases and review of the literature. J Clin Neurosci Off J Neurosurg Soc Australas. 2019; 62: 222-225.
- Bracard S, Schmitt E. Vasospasm and delayed consequences. Interv Neuroradiol J Peritherapeutic Neuroradiol Surg Proced Relat Neurosci. 2008; 14: 17-22.
- Chou SHY. Subarachnoid Hemorrhage. Contin Minneap Minn. 2021; 27: 1201-1245.
- Chukwudelunzu FE, Brown RD, Wijdicks EFM, Steckelberg JM. Subarachnoid haemorrhage associated with infectious endocarditis: case report and literature review. Eur J Neurol. 2002; 9: 423-427.
- Darsaut TE, Jack AS, Kerr RS, Raymond J. International Subarachnoid Aneurysm Trial - ISAT part II: study protocol for a randomized controlled trial. Trials. 2013; 14: 156.
- Ducruet AF, Hickman ZL, Zacharia BE, Narula R, Grobelny BT, Gorski J, et al. Intracranial infectious aneurysms: a comprehensive review. Neurosurg Rev. 2010; 33: 37-46.
- Habib G, Lancellotti P, Antunes MJ, Bongiorni MG, Casalta JP, Del Zotti F, et al. 2015 ESC Guidelines for the management of infective endocarditis: The Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio- Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur Heart J. 2015; 36: 3075-3128.
- Koos WT, Perneczky A, Auer LM, Boker DK, Gaab M, Jaksche H, et al. Nimodipine treatment of ischemic neurological deficits due to cerebral vasospasm after subarachnoid hemorrhage. Clinical results of a multicenter study. Neurochirurgia (Stuttg). 1985; 28: 114-117.
- Molyneux AJ, Kerr RSC, Yu LM, Clarke M, Sneade M, Yarnold JA, et al. International subarachnoid aneurysm trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised comparison of effects on survival, dependency, seizures, rebleeding, subgroups, and aneurysm occlusion. Lancet Lond Engl. 2005; 366: 809-817.
- Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP, Guyton RA, et al. 2014 AHA/ACC Guideline for the Management of Patients with Valvular Heart Disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014; 129: 2440-2492.
- Osgood ML. Aneurysmal Subarachnoid Hemorrhage: Review of the Pathophysiology and Management Strategies. Curr Neurol Neurosci Rep. 2021; 21: 50.
- Petruk KC, West M, Mohr G, Weir BK, Benoit BG, Gentili F, et al. Nimodipine treatment in poor-grade aneurysm patients. Results of a multicenter doubleblind placebo-controlled trial. J Neurosurg. 1988; 68: 505-517.
- Singla A, Fargen K, Blackburn S, Neal D, Martin TD, Hess PJ, et al. National treatment practices in the management of infectious intracranial aneurysms and infective endocarditis. J Neurointerventional Surg. 2016; 8: 741-746.
- Spetzler RF, McDougall CG, Zabramski JM, Albuquerque FC, Hills NK, Russin JJ, et al. The Barrow Ruptured Aneurysm Trial: 6-year results. J Neurosurg. 2015; 123: 609-617.
- Tjahjadi M, Heinen C, König R, Rickels E, Wirtz CR, Woischneck D, et al. Health-related quality of life after spontaneous subarachnoid hemorrhage measured in a recent patient population. World Neurosurg. 2013; 79: 296- 307.