
Case Report
Austin J Clin Case Rep. 2015;2(3): 1073.
Still Alive Three Years after Being Diagnosed with Inoperable Cholangiocarcinoma
Øistein Hovde1,2*, Mildrid Asgeirsdatter Ullerud³, Maiken Schultz4, Kjetil Weyde4, Oline Sofie Berget5, Krzysztof Wladslaw Grzyb6 and Sigmund Lavik7
¹Department of Gastroenterology, Innlandet Hospital Trust, Norway
²Department of Clinical Medicine, University of Oslo, Norway
³Department of Internal Medicine, Innlandet Hospital Trust, Norway
4Department of Oncology, Innlandet Hospital Trust, Norway
5Department of Radiology, Innlandet Hospital Trust, Norway
6Department of Pathology, Oslo University Hospital, Norway
7Department of Surgery, Innlandet Hospital Trust, Norway
*Corresponding author: Øistein Hovde, Department of Gastroenterology, Innlandet Hospital Trust, Gjøvik, Norway and Department of Clinical Medicine, University of Oslo, Norway
Received: March 31, 2015; Accepted: June 02, 2015; Published: June 03, 2015
Abstract
A 52 year old female with inoperable cholagiocarcinoma is presented. She was treated with two different chemotherapy regimens and a total of four stents (one stent in the common bile duct and three gastroduodenal stents). The cancer slowly progressed, but more than three years after inoperability was confirmed, she is still alive. Even if the clinical situation seems difficult, some patients live much longer than expected.
Introduction
Cholangiocellular Carcinoma (CCC) is the second most common primary hepatic malignancy. The incidence worldwide is rising, but the etiology is unknown.Numerous clinical trials have been conducted, and different chemotherapy regimens have been used, but still we have limited systemic therapeutic options for CCC.This neoplasm arises from the biliary epithelium and remains highly resistant to current chemoradiation therapies, but gemcitabine- and cisplatin - regimens are considered the first line therapy for patients with advanced disease. Other chemotherapy regimens that have been used are gemcitabine/oxaliplatin, gemcitabine/capecitabine, and 5-fluorouracil [1]. Complete surgical resection is the only chance for cure, but only about 10 % of the patients are candidates for surgery. Patients with unresectable or metastatic CCC have a median overall survival time of less than one year [1]. 5-year survival rate for CCC remains poor and five-year survival even in patients undergoing liver resection does not exceed 35 % [2]. Due to its rising incidence and the relatively poor prognosis there is an increasing focus on cholangiocarcinoma. Treatment options are chemotherapy, surgery, photodynamic treatment, or combinations of these options. The cause of death is usually the tumor burden, but biliary obstruction, leading to cholangitis and liver failure, is also a major cause. For that reason, relief of biliary obstruction is crucial in the treatment for patients with CCC. We present a relatively young patient with inoperable cholangiocarcinoma who is still alive more than three years later.
Case
A female patient, 52 years old, was admitted to the hospital 3 ½ years back with upper abdominal pain. Ultrasound examination revealed a moderately enlarged gallbladder with sludge. Cholecystectomy was performed. The bile ducts and surrounding tissue seemed to be inflamed. Perioperative cholangiography was consistent with cholangiocarcinoma. CT and MRI showed no signs of metastases. Laparoscopy was performed as a preparation for liver transplant. This examination revealed eight peritoneal lesions, each with a diameter of about one cm. Histological examination revealed infiltration of adenocarcinoma, biliary type, in the connective tissue and adipose tissue (Figures 1-2). In addition one lymph node from the area around the hepatic artery was removed, but no tumor tissue was found here. Two months later, because of jaundice and dilated biliary ducts, Endoscopic Retrograde Cholangiopancreatography (ERCP) was performed. A 3-4 millimeter long stricture in the common bile duct four cm from the papilla was found. Implantation of a 10 cm long and eight mm wide Self-Expanding Uncovered Metal Stent (SEMS) (WallFlex, Boston Scientific) was performed. Serum-bilirubin, which had been around 100 mmol/l, normalized, and the next month chemotherapy was started. A combination of gemcitabine and capecitabine was the first regimen used. The dosage for gemcitabine was 1000 mg/sqm on day 1 and 8 and capecitabine 625 mg/sqm were given twice daily every day for two weeks with a new cycle starting every third week. This treatment was given for eight months followed by a discontinuation of seven months when the same treatment was given for an additional month when the treatment, because of adverse effects, was stopped.Four months later gastroscopy for vomiting was done and a stenosis in duodenum was dilated and later stented; ultimately, three stents (duodenal stents, WallFlex, Boston scientific), were required because of tissue ingrowth (Figure 3). Two years after diagnosis and the next three months eight treatments with FLOX- (fluorouracil, leucovorin, oxaliplatin) regimen were given. The dosage was 5- fluorouracil 500 mg/sqm on day 1 and 2, calciumfolinate 100 mg on day 1 and 2, and oxaliplatin 85 mg/sqm on day 1. Three years after diagnosis the clinical situation deteriorated. CT and MRI (Figure 4) indicated progression of the disease with ascites and increased tumor metastases diffusely spread in the abdomen leading to surgery for small and large bowel obstruction. Due to tumor ingrowth it was impossible to restore bowel continuity with by-pass procedures. An ileostomy with placement of a deflating tube into an obstructed right colon and a gastrostomy were performed.In the same procedure permanent ascites drainage was established. Three weeks after the surgery the patient was discharged to her home. She is still alive 3 ½ years after diagnosis and is able to ski or walk one hour a day.
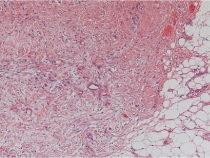
Figure 1: Tumor infiltration into the adipose and connective tissue.
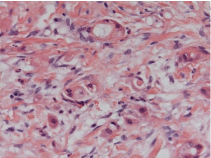
Figure 2: Glandular structures consistent with the diagnosis of
adenocarcinoma of biliary type.
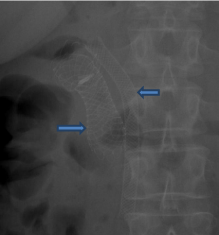
Figure 3: X-ray of the abdomen December 2014 demonstrating three stents
in duodenum (left arrow) and the stent in the common bile duct (right arrow).
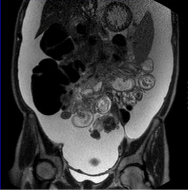
Figure 4: MRI January 2015 demonstrating tumor infiltrating the hilus region
of the liver, mesocolon, and the mesentrium of the small intestine. In addition:
Large amounts of ascites.
Discussion
The incidence of Cholangiocellular Carcinoma (CCC) is increasing and is now the second most common primary malignancy of the liver [3]. To date, there is no effective screening strategy. Therapy for CCC is challenging. Resection, when possible, is the preferred therapy. In selected cases liver transplantation might lead to a reasonable survival. Use of endoscopically directed brachytherapy with the aim of providing locoregional control in patients awaiting liver transplantation is occasionally used. A recent study including of four patients [4] showed that this is a well tolerated and safe procedure. Chemotherapy regimens consisting of gemcitabine and cisplatin are the preferred medical treatment options [5]. An interesting case from Japan [6] advocates sensitivity testing for anticancer drugs. In that case, based on this testing, a combination of cisplatin and S-1 (an oral fluoropyrimidine agent) was given. Two months into the second chemotherapy treatment, CT revealed a reduction of the tumors in the liver and lymph node and a reduction in pleural effusion. The patient was alive 17 months after the diagnosis of inoperable CCC.
One question raised is whether chemotherapy can improve survival. Most of the clinical experience comes from phase II studies [1]. A recent pooled analysis of clinical trials from 2000 to 2014 [7] demonstrated that gemcitabine combined with platinum compounds seems to be the best regimen concerning both response rate and tumor control rate. The conclusion from the authors was that gemcitabine combined with cisplatin or oxaliplatin is the most active standard regimen until a new evidence-based standard is defined [6]. Why do some patients respond better to a specific regimen than other patients? Is it simply a matter of plumbing with improved survival, a consequence of biliary and gastroduodenal luminal patency? Obviously the disease course, responsiveness to chemotherapy, and the molecular profiles differ. Chemosensitivity testing is a method for evaluation of tumor response prior to treatment with chemotherapy [7]. This method has, because of the poor prognosis and because the disease is relatively uncommon, seldom been used in CCC, but is an option we must bear in mind.
Conclusion
As this case illustrates, even when the situation might seem extremely difficult some patients might have a better prognosis than expected. Doctors treating patients with inoperable cholangiocarcinoma should be aware of the fact that some patients live much longer than one assume and that some optimistic information could be given to the patients.
References
- Hezel AF, Zhu AX. Systemic therapy for biliary tract cancers. Oncologist. 2008; 13: 415-423.
- Hemming AW, Reed AI, Fujita S, Foley DP, Howard RJ. Surgical management of hilar cholangiocarcinoma. Annals of Surgery. 2005; 241: 693-670.
- Yazici C, Niemeyer DJ, Iannitti DA, Russo MW. Hepatocellular carcinoma and cholangiocarcinoma: an update. Expert review of gastroenterology & hepatology. 2014; 8: 63-82.
- Cosgrove ND, Al-Osaimi AM, Sanoff HK, Morris MM, Read PW, Cox DG, et al. Photodynamic therapy provides local control of cholangiocarcinoma in patients awaiting liver transplantation. American Journal of Transplantation. 2014; 14: 466-471.
- Razumilava N, Gores GJ. Classification, diagnosis, and management of cholangiocarcinoma. Clinical Gastroenterology & Hepatology. 2013; 11: 13-21.
- Abe K, Wakatsuki T, Katsushima F, Monoe K, Kanno Y, Takahashi A, et al. A case of advanced intrahepatic cholangiocarcinoma successfully treated with chemosensitivity test-guided systemic chemotherapy. World Journal of Gastroenterology. 2009; 15: 5228-5231.
- Eckel F, Schmid RM. Chemotherapy and targeted therapy in advanced biliary tract carcinoma: a pooled analysis of clinical trials. Chemotherapy. 2014; 60: 13-23.