
Case Report
Austin J Clin Case Rep. 2016; 3(1): 1085.
Prolonged Pancytopenia in Chronic Urticaria Following Treatment with Cetirizine
Tajima K¹*, Sato S² and Kato T³
¹Department of Radiation Emergency Medicine, National Institute of Radiological Sciences, Japan
²Department of Neurology, Hematology, Metabolism, Endocrinology, and Diabetology, Yamagata University School of Medicine, Japan
*Corresponding author: Katsushi Tajima, Department of Radiation Emergency Medicine, National Institute of Radiological Sciences, National Institute of Sciences, 4-9-1, Anagawa, Inage-ku, Chiba-shi, Chiba, 263-8555, Japan
Received: February 01, 2016; Accepted: April 05, 2016; Published: April 07, 2016
Abstract
Cetirizine is considered as a safe treatment for allergic reactions such as an urticaria. We report for the first time a pancytopenia case treated with cetirizine for her chronic urticaria. We assessed a causality of the cetirizine reaction for pancytopenia using the Nranjo Scale, indicating probability. Cetirizine may have indistinctive hematological abnormalities, which should be kept in mid in cases of treatment with this drug.
Keywords: Cetirizine; Chronic urticaria; Pancytopenia; The Naranjo Algorithm
Case Presentation
A 23-year-old woman received hydroxyzine (100 mg/day) and tranexamic acid (750 mg/day) for 3 years as treatment for chronic idiopathic urticaria without blood cell abnormalities. She had no medical history of asthma or collagen disease. The urticaria persisted despite treatment with these drugs. She was given cetirizine 10 mg/ day. Her laboratory data included: white blood cell (WBCs) 5900/mm3 with 56% neutrophils, hemoglobin (Hb) 12.2 g/dL, platelets (PLt) 243000/mm³, and aspartate aminotransferase (AST) 38 IU/L. Her serum IgE was 526 U/ml (normal< 250). Viral antibodies for hepatitis C and B were negative. Two weeks after treatment with cetirizine, she developed thrombocytopenia (45000/mm3) and leukopenia (2400/ mm3). She had no cold symptoms and received no additional drugs during this period. Cetirizine treatment was stopped quickly, and 2 weeks later, she was transferred to our hospital for further evaluation.
She presented with petechial hemorrhages and urticaria on the limbs without hepatosplenomegaly, lymph node swelling, or fever. The laboratory data included: WBC 2170 with 5% neutrophils (110/ mm3), PLt (21000/mm3), and Hb (10.0 g/dL: reticulocytes 0.67%). Her serum complement and C-reactive protein were normal, and serum erythropoietin increased to 1190 mU/mL (normal: 8-36). Flow cytometry analysis revealed no paroxysmal nocturnal hemoglobinuria clonal blood cells. Bone marrow aspirate exhibited hypocellularity with a lack of erythroid precursors and few megakaryocytes without dysplasia. A bone marrow biopsy revealed hypoplasia with severe fatty changes. Magnetic resonance imaging of the spine showed fatty changes in the bone marrow. Extensive examination, including tests for IgM antibody against parvovirus B19 and its DNA in the serum and bone marrow, and various collagen disease-related markers indicated no specific cause for her pancytopenia. Based on these findings, she was diagnosed with severe aplastic anemia.
She refused antithymocyte globulin or cyclosporine therapy for her aplastic anemia. We could not rule out the possibility that cetirizine administration induced her pancytopenia, and thus she was monitored by observation, except for minimal Plt and red blood cell transfusions (Figure 1). Her anemia and thrombocytopenia slowly recovered over 10 weeks of watchful waiting (Figure 1). Finally, a causality assessment of the adverse drug reaction using the Naranjo Algorithm scored a 6, indicating probability for pancytopenia due to treatment with cetirizine [1].
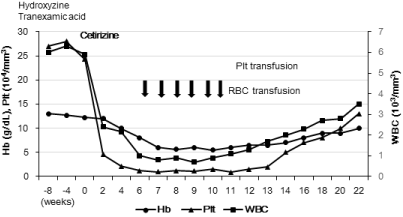
Figure 1: Course of pre-and post-treatment with cetirizine and laboratory
data.
Discussion
Cetirizine, a hydroxyzine metabolite, is a reversible selective histamine-1 receptor antagonist belonging to the second-generation piperazine class, and is established as a highly effective and safe treatment for allergic reactions. Although no presently available drug is completely free from adverse effects [2], cetirizine-induced pancytopenia, however, has not been previously described in the literature.
Recent studies shed new light on the interactions between histamine receptors and hematopoiesis [3-6]. Tan et al. suggested that histamine-1 and-2 receptors are present on hematopoietic cells, and histamine-2 receptor agonists increase the number of colony-forming unit granulocyte macrophages and megakaryocytes [6]. A recent study demonstrated that through the histamine-1 receptor, bone marrow stromal cells produce interleukin-6 and -8, which enhance chemo attractants and survival of neutrophils, and hitstamine-1receptor antagonists block this cytokine production [5]. Hydroxyzine also belongs to the first-generation histamine-1 receptor antagonist with anticholinergic effect, whereas hydroxyzine has induced no pancytopenia for a prolonged period. Her pancytopenia may not be due to histamine-1 receptor related functions but to metabolic differences or metabolite profiling between them.
On the other hand, pancytopenia associated with virus infection is well known, and this pancytopenia except for parvovirus B19 infection recovers along with improvement of virus infection, not really consistent with prolonged her pancytopenia as well as no evidences of infection diseases or symptoms. It is difficult to directly prove a causal relationship between cetirizine administration and pancytopenia without rechallenge cetirizine treatment, which involves risk and ethical problems. Although the precise mechanism by which cetirizine causes pancytopenia is unknown, however, the Naranjo Algorithm showed a probability associated with cetirizine in this case.
In conclusion, cetirizine may have undetermined effects on the hematopoietic system. Therefore, blood cell abnormalities, including pancytopenia, should be kept in mind when prescribing this drug and watchful waiting as the treatment should be considered to differentiate an adverse drug effect from idiopathic aplastic anemia.
References
- Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, Roberts EA, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981; 30: 239-245.
- Routledge PA, Lindquist M, Edwards IR. Spontaneous reporting of suspected adverse reactions to antihistamines: a national and international perspective. Clin Exp Allergy. 1999; 3: 240-246;
- Corbel S, Schneider E, Lemoine FM, Dy M. Murine hematopoietic progenitors are capable of both histamine synthesis and uptake. Blood. 1995; 86: 531-539.
- Kohka H, Nishibori M, Iwagaki H, Nakaya N, Yoshino T, Kobashi K, et al. Histamine is a potent inducer of IL-18 and IFN-gamma in human peripheral blood mononuclear cells. J Immunol. 2000; 164: 6640-6646.
- Nemeth K, Wilson T, Rada B, Parmelee A, Mayer B, Buzas E, et al. Characterization and function of histamine receptors in human bone marrow stromal cells. Stem Cells. 2012; 30: 222-231.
- Tan M, Pan Z, Wang Q, Xu Y. Effects of different subtypes of histamine receptors on proliferation and differentiation of murine colony forming unit granulocyte-macrophage and colony forming unit megakaryocyte. Chin Med J (Engl). 1998; 111: 132-135.