
Research Article
Austin J Clin Case Rep. 2016; 3(3): 1093.
Papillary Renal Cell Carcinoma Cytological-Histological Correlation and Diagnostic Pitfalls
Pinnamaneni R¹, Ahmad N¹, Khalil FK³, Bui MM4 and Henderson-Jackson EB3*
¹University of South Florida, USA
²James A Haley VA Hospital, USA
³Department of Anatomic Pathology, H. Lee Moffitt Cancer Center & Research Institute, USA
4Departments of Anatomic Pathology and Sarcoma, H. Lee Moffitt Cancer Center & Research Institute, USA
*Corresponding author: Evita B Henderson-Jackson, Department of Anatomic Pathology, H. Lee Moffitt Cancer Center & Research Institute, 12902 Magnolia Drive, Tampa FL 33612, USA
Received: May 03, 2016; Accepted: August 31, 2016; Published: September 05, 2016
Abstract
The papillary variant of renal cell carcinoma has distinctive cytological, histological, and clinical features. There are a few studies describing its cytological features. We describe the cytological and correlating histological features of two cases of papillary renal cell carcinoma, type-2. The smears revealed papillary-like clusters with relatively few single cells. The first case had tumor cells with scant to moderate cytoplasm, small to medium-sized nuclei, single and small nucleoli, and occasional nuclear grooves. Foamy macrophages were rare. Whereas, the second case had tumor cells that exhibited moderate cytoplasm with prominent vacuolization, larger nuclei with mild to moderate pleomorphism, and conspicuous nucleoli. Psammona bodies were numerous in one case while absent in the other. Subsequent histology and immunohistochemical stains confirmed the diagnosis of papillary renal cell carcinoma, type-2. Papillary renal cell carcinoma is a type of renal carcinoma that can often be accurately diagnosed by fine-needle aspiration. Cytologically recognizing this papillary tumor is important in the clinical management of patients.
Keywords: Papillary; Renal cell carcinoma; Fine needle aspiration
Introduction
Papillary renal cell carcinoma (PRCC) is a rare type of renal malignancy representing 7%-15% of renal carcinomas [1,2]. By histology, PRCC is defined as a malignant epithelial tumor of the kidney with a minimum of 50% papillary architecture. Delahunt and Eble [3] reported the existence of two subtypes of PRCC with distinct morphologic features. PRCC type-1 is composed of papillae covered by a single or double layer of small cells with scanty, pale cytoplasm. The cells possess small ovoid nuclei with inconspicuous nucleoli. PRCC type-2 is made up of papillae covered by cells with large spherical nuclei, prominent nucleoli, and abundant eosinophilic cytoplasm arranged in a pseudostratified manner [3].
The prognosis of patients with PRCC differs from that of patients with other variants of renal cell carcinoma. Papillary renal tumors are not as aggressive as renal cell carcinoma, clear cell type, but PRCC type-2 tend to be more aggressive and thus carry a worse prognosis than type-1 PRCC [2-5]. Fine-needle aspirations (FNA) of renal masses are performed to assess patient management which can result in resection if appropriate. Thus, the distinction of a specific variant of renal carcinoma may be crucial to the patient outcome. Recognition of pertinent cytological features of PRCC will allow for accurate diagnosis and the ability to differentiate other primary renal neoplasms and metastatic papillary tumors from PRCC. There have been a few reports describing the FNA diagnosis of PRCC [6-8]. In this report, we describe two cases of papillary renal cell carcinoma.
Materials and Methods
Two cases of papillary renal cell carcinoma were reviewed. (Case 1) Specimen was obtained by ultrasound guided fine-needle aspiration (FNA) biopsy using 22-gauge needle. (Case 2) Specimen was obtained by CT-guided FNA biopsy using 22-gauge needle. Both air-dried and ethanol-fixed smears were prepared. The air-dried specimens were stained with Diff-Quik and the ethanol-fixed slides were stained with Papanicolaou method. Cell blocks were made and fixed in formalin and embedded in paraffin. Sections were cut (5 um) and stained with hematoxylin and eosin. Additional sections were cut from the cell block material for immunohistochemical stains. Appropriate positive and negative controls were used.
Case 1
A 59 year-old gentleman presented with a 2.6 cm hyperdense left renal mass. Additional CT scan findings showed a 5.1 cm left para-aortic soft tissue mass and a 1.4 cm enlarged right paratracheal lymph node. The patient stated that he had increased frequency with urination at night. He denied any abdominal pain.
A FNA of the left renal mass and the left para-aortic soft tissue mass was performed. Cytologic smears were cellular and composed of numerous three-dimensional papillary groups of cells, as well as, single cells in the background (Figure 1a). The malignant cells demonstrated minimal-mild nuclear pleomorphism. Rare small nucleoli and occasional nuclear grooves were noted. The tumor cells had scant-to-moderate amount of cytoplasm with occasional cytoplasmic vacuolization. Numerous psammoma bodies were present (Figure 1b). Foamy macrophages were seen in the background. Based on these findings, the diagnosis of papillary renal cell carcinoma was rendered. Immunohistochemical stains done on the cell block were positive for cytokeratin 7, AMACR, and vimentin (Figure 2). In addition, the tumor was negative for CD10 and cytokeratin 20. The cytologic smears from the left para-aortic soft tissue mass demonstrated similar findings.
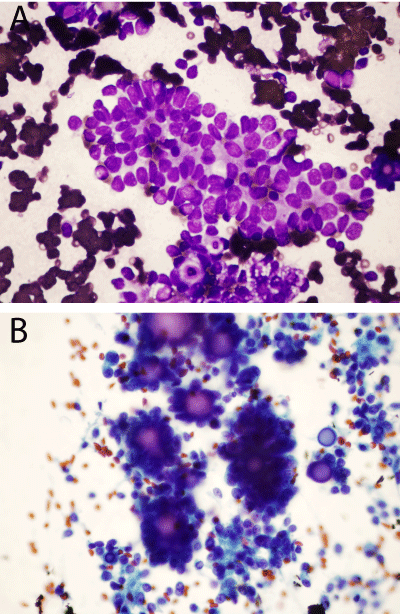
Figure 1: Case 1 FNA: (a) Three dimensional papillary groups with
occasional nuclear grooves and adjacent tumor cells with occasional
cytoplasmic vacuolization [Diff Quik, 40X] (b) Numerous psammona bodies
[Pap stain, 40X].
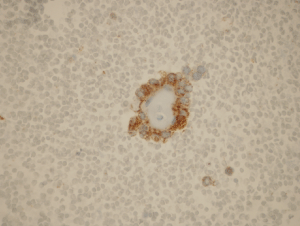
Figure 2: Case 1 AMACR immunostain: Tumor cells express AMACR immunostain [40x].
A total nephrectomy performed a couple of months later, confirmed the cytologic diagnosis of papillary renal cell carcinoma. The tumor measured 3.9 cm in greatest dimension surrounded by a thick fibrous capsule. It was determined to be of subtype 2 consisting of greater than 50% papillary structures covered by cells with abundant eosinophilic cytoplasm and prominent nucleoli arranged in a stratified manner (Figure 3a-3b). There were extensive areas of necrosis in the tumor. Numerous psammona bodies were seen. In addition, there were focal areas of the tumor demonstrating sarcomatoid features. The para-aortic soft tissue mass measured 7.0 x4.6 x4.0 cm and was determined to be a lymph node completely replaced by tumor.
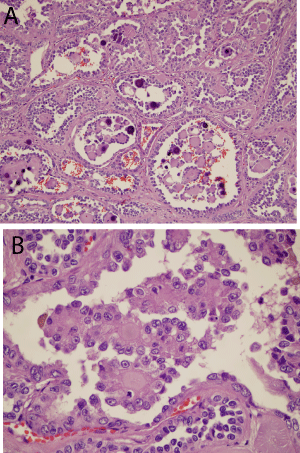
Figure 3: Case 1 Histology: (a)Low power view of the papillary structures
and psammona bodies [Hematoxylin and Esoin, 10X] (b)Papillary structures
covered by stratified epithelium containing nuclei with prominent nucleoli and
abundant eosinophilic cytoplasm [Hematoxylin and Eosin, 40X].
Case 2
A 74 year-old gentleman with a history of metastatic melanoma presented with an enhancing lesion in his left kidney concerning for an additional metastasis. CT scan of the abdomen revealed a well- defined, solid, hypovascular cortically based mass in the upper pole of the left kidney measuring 2.1 x 2.0 cm. The imaging findings were not classically consistent with a hypervascular metastasis or clear cell renal cell carcinoma.
A CT-guided FNA of the left renal mass was performed. Cytologic smears were cellular and comprised of three-dimensional papillary groups of cells. Single cells were scattered in the background. The malignant cells had enlarged nuclei with mild to moderate nuclear pleomorphism and prominent nucleoli. Tumor cells had moderate amount of cytoplasm exhibiting significant vacuolization (Figure 4a- 4b). Smaller proportion of tumor cells had eosinophilic cytoplasm. Psammoma bodies were absent. Numerous foamy macrophages were present in the background. Immunohistochemical stains performed on the cell block were positive for RCC while negative for CD10, HMB-45 and Melan-A. Based on these findings, a diagnosis of clear cell renal cell carcinoma was rendered.
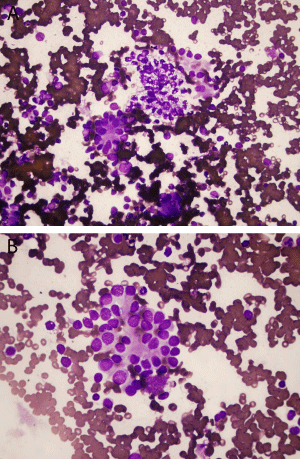
Figure 4: Case 2 FNA: (a) Three dimensional papillary groups, scattered
single cells, and focal cytoplasmic vacuolization of neoplastic cells [Diff Quik,
20X] (b) Tumor cells with moderate eosinophilic cytoplasm and enlarged
nuclei with mild nuclear pleomorphism [Diff Quik, 40X].
A partial nephrectomy was performed a month later. The 2.2 cm mass was proven to be a papillary renal cell carcinoma, subtype 2. Grossly, the tumor was surrounded by thin capsule and cut surfaces were tan-yellowish in color and partially hemorrhagic and necrotic. Histological sections of the tumor demonstrated papillary-solid architecture of with the papillae were closely packed, masking their true papillary growth pattern. Delicate, thin fibrovascular cores are identified surrounded by polygonal cells with predominantly eosinophilic cytoplasm however; focally the cells exhibited cytoplasmic clearing. Nuclei of the tumor cells were round to elongate with nuclear membrane irregularities and conspicuous nucleoli (Figure 5). Scattered foamy macrophages and geographic necrosis were present. Psammona bodies were not identified.
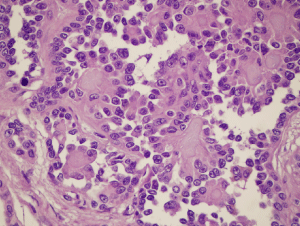
Figure 5: Case 2 Histology: Closely packed papillary structures lined by tumor
cells with round to elongated nuclei with nuclear membrane irregularities and
conspicuous nucleoli [Hematoxylin and Eosin, 40X].
Discussion
Papillary renal cell carcinoma (PRCC) constitute 7%-15% of renal epithelial tumors and possess characteristic gross, histologic, and cytogenetic features [1,2]. A high proportion of PRCCs are organ-localized at the time of diagnosis [2,9]. Type-1 PRCCs tend to be localized to the kidney, whereas type-2 PRCCs frequently present with locally invasive disease [9]. PRCCs have characteristic radiologic findings due to the fact that they are often hypodense, cystic and necrotic appearing on CT scans. Angiographically, PRCC often appear avascular or hypovascular [2]. Grossly, the majority of PRCCs are reported as being well-circumscribed, often with thick fibrous capsules and multifocality appears to be a prominent feature [4,5,10]. Microscopically, PRCCs have two distinct morphological types of tumor and type-2 PRCCs usually have less favorable features in comparison with type-1 PRCCs. Type-1 PRCCs characteristically show mild-moderate nuclear pleomorphism [3,11,12] and rare mitotic figures. This subtype is less likely to metastasize or show vascular invasion. On the other hand, type-2 PRCCs usually present with moderate-marked nuclear pleomorphism [3,11, 12]. The presence of foamy macrophages within the papillary cores appears to be common in type-1 PRCCs and rarely noted in type-2 tumors. Tumor necrosis, hemorrhage, and inflammatory infiltrates are common in both subtypes [3,5]. Cytogenetic studies frequently demonstrate trisomies for chromosome 7 and 17, usually in type-1 tumors, and X chromosome losses. Xp losses has reportedly been associated with poor patient prognosis [13]. Immunohistochemically, type-1 PRCCs reportedly show strong expression of cytokeratin 7 [3,9] but, another report showed a decrease of expression of cytokeratin 7 in type-1 PRCCs and an increased expression in type-2 PRCCs [11]. Recently, the immunohistochemical stain N-cadherin has been identified as a marker for the differentiation between PRCC type-1 and PRCC type- 2 [14].
Type-2 PRCCs often present at a more advance stage than type- 1 tumors [3,12,15] and appear to be common in patients younger than age 40 [12]. The 5 year cancer-specific survival appears to be significantly worse for type-2 tumors compared to type-1 tumors (50% vs. 94%), respectively [15]. Overall, 5 year survival rate of patients with PRCC is 82%-90% which is better than that of patients with clear cell carcinoma (65%-75%) [5].
It is important for the pathologist/cytopathologist to be familiar with the cytologic features of PRCC, in that, it may have important prognostic and therapeutic indications. Trying to further subtype a PRCC into type-1 versus type-2 at this point is not critical. Fine needle aspirates of PRCCs have cellular smears with numerous tridimensional papillary fragments with fibrovascular cores [6,7]. The fragments are seen in cohesive groups with rare single tumor cells in the background and psammona bodies may be seen [7]. The cells usually contain small, ovoid nuclei with small inconspicuous nucleoli, and slight-to-moderate hyperchromasia [7]. Unlike papillary tumors, Dekemizian et al. [7] had described clear cell RCC as being composed of loosely cohesive groups of cells with numerous single tumor cells and frequent stripped nuclei in the background. Most cells show an open chromatin pattern with prominent nucleoli and abundant cytoplasm. Altogether, Dekmezian et al. [7] study reported that the most distinguishing features of PRCCs were nuclear with frequent nuclear grooves, indistinct nucleoli, and rare nuclear pleomorphism. Psammona bodies may help in the diagnosis. They are reported to be present in approximately 40% of PRCCs in a study by Manciella et al. [1].
Granter et al. [6] reported in their series that the most sensitive features for diagnosing PRCC were foamy macrophages (82%) and intracytoplasmic hemosiderin in tumor cells (76%). The specificity was 96% for both features. Nuclear grooves were seen in 9 of 17 cases (53%). Of note, Granter et al. [6] noted that the presence of fingerlike papillae with bulbous, rounded ends and spherules appear to be a more specific feature of PRCC and was not observed in any of their 40 cases of clear cell RCC. Papillary structures in the clear cell RCCs when identified possess ragged, irregular outlines.
The cytological smears from our cases demonstrated increased cellularity and three-dimensional cohesive papillary groups with rare single tumor cells in the background. Psammona bodies were present within case 1 while absent in case 2. Foamy macrophages were numerous in case 1 while rarely seen in case 2. Nuclear pleomorphism was mild in case 1 and moderate to marked in case 2. Nuclear grooves were seen in case 1. However, both cases revealed tumor cells with prominent nucleoli and moderate eosinophilic cytoplasm. Cytoplasmic clearing was more prominent in case 2 than case 1 and the thin fibrovascular cores in case 2 retrospectively, can be easily mistaken for thin-walled vessels leading to a diagnosis of clear cell renal cell carcinoma.
One of the most common pitfalls in FNA cytology of renal tumors was the misclassification of papillary or sarcomatoid RCC as clear cell RCC as reported by Renshaw [16]. As seen in our case 2 which was diagnosed as clear cell renal cell carcinoma on FNA cytology. The main differential diagnoses include clear cell renal cell carcinoma (CCRCC) and clear cell papillary renal cell carcinoma (CCPRCC). This may be difficult due to the fact that CCRCCs and CCPRCCs may exhibit papillary or pseudopapillary architecture as well as cystic areas and PRCCs may demonstrate clear cells. A helpful feature of CCRCC is the delicate sinusoidal vascular network which is absent in PRCCs. CCPRCC neoplastic cells exhibit minimal to mild nuclear pleomorphism with nuclei oriented away from the basement membrane and toward the apical surfaces [17,18]. Immunohistochemically, CCRCC is usually immunoreactive for CD10 and RCC while negative for cytokeratin 7. Characteristically, CCPRCC are diffusely positive for cytokeratin 7 and negative for alpha-methylacyl-CoA racemase (AMACR) and CD10 [19]. PRCC is positive for cytokeratin 7 similar to CCPRCC, but PRCC is also positive for CD10 and AMACR. Renshaw et al. [16] concluded that FNA correctly identified the variant of RCC in 74% of cases in that series. This case report further illustrates the importance of recognizing the cytological features of PRCC, in order to differentiate it from clear cell renal cell carcinoma and clear cell papillary renal cell carcinoma.
References
- Blath RA, Mancilla-Jimenez R, Stanley RJ. Clinical comparison between vascular and avascular renal cell carcinoma. J Urol. 1976; 115: 514-519.
- Mydlo JH, Bard RH. Analysis of Papillary Renal Adenocarcinoma. Urology. 1987; 30: 529-534.
- Delahunt B, Eble JN. Papillary Renal Cell Carcinoma: A Clinicopathologic and Immunohistochemical Study of 105 Tumors. Modern Pathology. 1997; 10: 537-544.
- Renshaw AA, Corless CL. Papillary Renal Cell Carcinoma: Histology and Immunohistochemistry. Am J Surg Pathol. 1995; 19: 842-849.
- Amin MB, Corless CL, Renshaw AA, Tickoo SK, Kubus J, Schultz DS. Papillary (Chromophil) Renal Cell Carcinoma: Histomorphologic Characteristics and Evaluation of Conventional Pathologic Prognostic Parameters in 62 Cases. Am J Surg Pathol. 1997; 21: 621-635.
- Granter SR, Perez-Atayde AR, Renshaw AA. Cytologic Analysis of Papillary Renal Cell Carcinoma. Cancer. 1998; 84: 303-308.
- Dekmezian R, Sneige N, Shabb N. Papillary Renal-Cell Carcinoma: Fine-Needle Aspiration of 15 Cases. Diagn Cytopathol. 1991; 7: 198-203.
- Lim JC, Wojcik EM. Fine-Needle Aspiration Cytology of Papillary Renal Cell Carcinoma: The Association with Concomitant Secondary Malignancies. Diagn Cytopathol. 2006; 34: 797-800.
- Delahunt B, Eble JN, McCredie MR, Bethwaite PB, Stewart JH, Bilous AM. Morphologic Typing of Papillary Renal Cell Carcinoma: Comparison of Growth Kinetics and Patient Survival in 66 Cases. Hum Pathol. 2001; 32: 590-595.
- Waldert M, Haitel A, Marberger M, Katzenbeisser D, Ozsoy M, Stadler E, et al. Comparison of Type I and II Papillary Renal Cell Carcinoma (RCC) and clear cell RCC. BJU Int. 2008: 102: 1381-1384.
- Mydlo JH, Weinstein R, Misseri R, Axiotis C, Thelmo W. Radiologic, Pathologic and Molecular Attributes of Two Types of Papillary Renal Adenocarcinomas. Scand J Urol Nephrol. 2001; 35: 262-269.
- Kosaka T, Mikami S, Miyajima A, Kikuchi E, Nakagawa K, Ohigashi T, et al. Papillary Renal Cell Carcinoma: Clinicopathological Characteristics in 40 Patients. Clin Exp Nephrol. 2008; 12: 195-199.
- Jiang F, Richter J, Schraml P, Bubendorf L, Gasser T, Sauter G, et al. Chromosomal Imbalances in Papillary Renal Cell Carcinoma: Genetic Differences between Histological Subtypes. Am J Pathol. 1998; 153: 1467-1473.
- Behnes CL, Hemmerlein B, Strauss A, Radzun HJ, Bremmer F. N-cadherin is differentially expressed in histological subtypes of papillary renal cell carcinoma. Diagn Pathol. 2012; 7: 95.
- Yamashita S, Ioritani N, Oikawa K, Aizawa M, Endoh M, Arai Y. Morphological Subtyping of Papillary Renal Cell Carcinoma: Clinicopathological Characteristics and Prognosis. Int J Urol. 2007; 14: 679-683.
- Renshaw AA, Lee KR, Madge R, Granter SR. Accuracy of Fine Needle Aspiration in Distinguishing Subtypes of Renal Cell Carcinoma. Acta Cytol. 1997; 41: 987-994.
- Gobbo S, Eble JN, Grignon DJ, Martignoni G, MacLennan GT, Shah RB, et al. Clear cell papillary renal cell carcinoma: a distinct histopathologic and molecular genetic entity. Am J Surg Pathol. 2008; 32: 1239-1245.
- Bing Z, Tomaszewski J. Clear cell papillary renal cell carcinoma in the bilateral native kidneys after 2 years of renal transplantation: report of a case and review of literature. Case Reports in Transplantation. 2011: 1-4.
- Aydin H, Chen L, Cheng L, Vaziri S, He H, Ganapathi R, et al. Clear cell tubulopapillary renal cell carcinoma: a study of 36 distinctive low-grade epithelial tumors of the kidney. Am J Surg Pathol. 2010; 34: 1608-1621.