
Case Report
Austin J Clin Case Rep. 2016; 3(4): 1102.
Lymphoepithelioma-Like Carcinoma of the Breast: A Singular Morphological Pattern with an Expected Outcome
Herrera-Goepfert R1*, Caro-Sánchez C1 and Maafs-Molina E2
1Department of Pathology, Instituto Nacional de Cancerología, Mexico
2Oncology Clinic, Hospital Ángeles Clínica Londres, Mexico
*Corresponding author: Roberto Herrera-Goepfert, Department of Surgical Pathology, Instituto Nacional de Cancerología (INCan), Mexico
Received: August 23, 2016; Accepted: November 01, 2016; Published: November 16, 2016
Abstract
Lymphoepithelioma-Like (LEL) carcinoma of the breast is a rare variant of primary epithelial cancer with a favorable prognosis, with long-term survival, and low rate of metastasis and/or local recurrence. We report the case of a 54 year-old woman who developed an LEL carcinoma in her left breast. Neoplastic cells were positive for cytokeratin cocktail, Epithelial Membrane Antigen (EMA), estrogen, and progesterone receptors, and GATA 3 and negative for Epstein- Barr Virus (EBV) In Situ Hybridization (ISH), E-cadherin, and β-catenin, among others. E-cadherin and β-catenin status has not been previously addressed in LEL carcinomas of the breast. We wonder whether LEL carcinoma of the breast could be considered as a distinct immunogenic/molecular variant of invasive lobular or ductal carcinoma, or whether it should be considered as a distinct neoplastic entity. Further studies are warranted in order to clear up the origin and clinico-pathologic features of this singular type of breast carcinoma.
Keywords: Lymphoepithelioma-like carcinoma; Breast; E-cadherin; β-catenin; Epstein-Barr virus
Introduction
Breast carcinoma comprises a set of malignant epithelial neoplasms with diverse morphologic, genetic, and molecular features, which impose different clinical, prognostic and therapeutic approaches; ductal and lobular carcinomas are the most frequent invasive histological types. Lymphoepithelioma-Like (LEL) carcinoma -first described by Kumar and Kumar in 1994 as a lobular carcinoma- is a rare variant with to our knowledge no >22 cases reported in the English-language literature, including the present case [1]. However, at present agreement is lacking with regard to its histological phenotype; thus, it has been categorized as an undifferentiated carcinoma, as in other anatomical regions [2]. Conversely with respect to other types of breast carcinoma, LEL carcinoma of the breast has a favorable prognosis; in general terms, it is a local disease, with longterm survival, and a low rate of metastasis and/or local recurrence. We report the case of a woman who developed a lobulated tumor in her left breast that was finally diagnosed as a primary LEL carcinoma.
Material and Methods
A nulliparous and menopausal 57-year-old female patient arrived at an outpatient clinic at her place of origin on November 2015, due to a self-detected tumor in her left breast. The patient had a history of a breast lump in the opposite breast 12 years previously, with a diagnosis of sclerosing adenosis and she had a sister harboring breast cancer. Since that time, she had been mammographically screened, with a recent study 1 year previously, negative for breast lesions. On clinical examination, a tumor of about 4 cm in diameter, nodular and mobile, was detected; there were no suspicious axillary lymph nodes for metastasis. At that time, mammography and ultrasonography revealed a dense, solid and hypoechoic tumor, with blurred borders, about 3 x 2.7 cm in size (Breast Imaging-Reporting Data System [BI-RADS IV]) (Figure 1). An incisional biopsy was performed. An undifferentiated carcinoma was diagnosed, after a CKAE1/AE3 positive immunohistochemical reaction. The patient was referred to an Oncology clinic in Mexico City. Clinically, the patient had a residual breast nodular lump in the left breast, without cervical or axillary adenopathy. The new mammographic study revealed a residual neoplastic tissue, highly suspicious of malignancy, with no evidence of calcifications, and classified as BI-RADS VI. In the meanwhile, a second histopathological opinion was requested on the previously excised material; an LEL breast carcinoma was diagnosed.
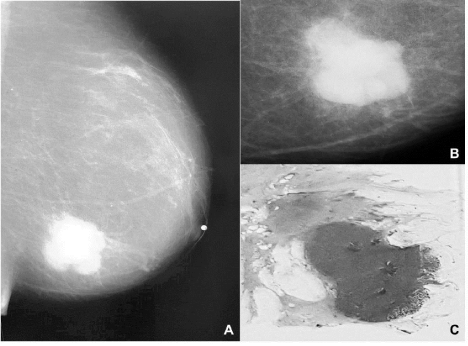
Figure 1: (A, B). Mammography images of the left breast show a solid, dense
tumor with blurred margins. A panoramic photograph of a section from the
tumor reveals a nodular, solid, and dense tumor, with pushing borders (C).
Hematoxylin & Eosin [H&E].
The tumor cells were arranged in non-syncytial cords, nests and dispersed cells, surrounded by dense inflammatory infiltrate of mature round lymphocytes and occasional plasma cells, histiocytes, and eosinophils. The cells exhibited pale cytoplasm, vesicular nuclei, and prominent nucleolus, with occasional atypical mitotic figures (Figure 2); in addition, focal necrosis was found. Immunohistochemical analysis was performed using the Mach 4 Universal detection system, on a BenchMark ULTRA autostainer (Ventana Medical Systems, Inc.), according to supplier protocol. The neoplastic cells were positive for CKAE1/AE3 (1:50; BIOGENEX), GATA 3 (1:50; BioSB), Epithelial Membrane Antigen (EMA) (1:50; DAKO), and estrogen receptors (H-score: 90), progesterone receptors (H-score 180), HER- 2 negative (1+) (Ventana protocol), 20% for proliferative marker Ki-67 (1:50; BioSB), and 60% for p53 (1:50; DAKO), in addition to lymphoid markers CD20 (1:400; DAKO), CD3 (1:150; DAKO), and CD8 (1:25; BIOCARE). Interestingly, the neoplastic cells were negative for E-cadherin (1:50; DAKO), and β-catenin (1:100; BioSB) (Figure 3). Epstein-Barr Virus (EBV) search was carried out by means of EBER in situ hybridization (BIOGENEX); it was also negative. All reactions were revealed using Diaminobenzidine (DAB) as developer. A Madden modified radical mastectomy was performed, with sentinel lymph-node biopsy, which did not demonstrate neoplastic cells. The pathological examination of the mastectomy specimen showed the same tumor characteristics with 2.1 cm as its largest dimension. Currently, the patient has no evidence of any tumor activity; she is under a chemotherapy treatment. Her follow-up imaging studies are negative for any tumor activity.
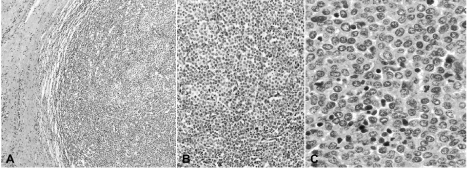
Figure 2: (A). A histological section from the tumor showing well defined and pushing tumor borders (H&E, 5X). (B). Large and small sheets and nests of malignant
epithelial cells are surrounded by lymphoid cells (H&E; 20X). (C). A solid sheet of intermediate- and small-sized neoplastic cells is intermingled with lymphocyte
infiltrates (H&E; 40X).

Figure 3: (A). Lymphoid generic marker intensely highlights the lymphoid cells immersed within the tumor cells. (B). Neoplastic cells display positivity for estrogen
receptor, and (C). Total absence of the marker for E-cadherin in neoplastic cells.
Discussion
Lymphoepithelioma (undifferentiated carcinoma) was simultaneously but independently described in the nasopharyngeal region by Regaud and Reverchon, and Schmincke, in 1921 [3]. This unique type of carcinoma is closely associated with Epstein- Barr Virus (EBV) infection in this region, and has been described in practically all anatomic regions. Tumors arising outside of the nasopharynx are now denominated LEL carcinomas. In addition to the nasopharynx, EBV is present in variable proportions of gastric, salivary gland, thymus, and lung carcinomas (foregut-derived organs), and attempts to demonstrate viral infection in many of the other sites are unforeseeable, with negative results in the majority of them. Originally, nasopharyngeal lymphoepithelioma carcinoma was described as having two histopathological patterns; the syncytial-type or Regaud-type, with closely packed neoplastic cells surrounded by reactive lymphocytes, and the non-cohesive pattern or Schmincketype, with nests, cords and dispersed neoplastic cells intermingling with reactive lymphocytes; in the nasopharynx, both of these are associated with EBV- infection. The majority of LEL carcinomas described in the human body correspond to the so-called Regaud-type. Regardless of anatomic location and/or histological appearance, the most striking feature comprises a dense, mononuclear inflammatory infiltrate. To the best of our knowledge, among the reported cases in which EBV status was intentionally investigated, no single case of LEL carcinoma of the breast, including the present case, has been positive for EBV, either by means of ImmunoHistoChemistry (IHC), In Situ Hybridization (ISH) and Polymerase Chain Reaction (PCR), thus excluding the role of EBV-associated malignant transformation. In a recent meta-analysis [4], EBV was only related with lobular and ductal breast carcinoma in 29% of pooled patients, with a trend toward strongest association with lobular carcinoma and an increased risk for breast Carcinoma. On the other hand, two cases of LEL breast carcinomas have been related with Human Papilloma Virus (HPV) infection, one by ISH and PCR, and the other by ISH but absent by PCR [5,6]. We have previously stated that the presence of HPV signals in neoplastic tissue does not necessarily mean a causal relationship, because HPV -infection could take place after the development of the carcinoma [7]. It is noteworthy that the patient reported by Kulka et al. had a previous history of cervical carcinoma [4]. Twenty -one cases of LEL carcinomas of the breast have been previously published, the majority of these as case reports, in women with a median age of 54 years (range, 37–69 years), with tumors ranging from 1-4 cm in size and with lymph node metastasis in 25% of cases. Estrogen and progesterone receptors have been positive in 47 and 26% of the cases, respectively, and HER-2 receptor status was found to be over expressed in 3 of 15 patients in whom the search was performed [8]. An interesting but not very well known issue is that related with the phenotype of the neoplastic cells. Usually, the term “undifferentiated” implies a neoplasm with dismal prognosis and of unknown origin. However, LEL carcinomas originate in organs other than nasopharynx and have a prognosis that is not as harmful as other types of carcinomas in the same region. In the case of the mammary gland, some authors have considered LEL carcinoma as a variant of lobular carcinoma. Kumar and Kumar described in situ and infiltrating lobular carcinoma in the same excised mammary gland, as did Cristina et al. and Pestereli et al. whereas Sanati et al. described atypical lobular hyperplasia [2,9-11]. To the best of our knowledge, E-cadherin and β-catenin status has not been previously addressed in LEL carcinomas of the breast, and in our case, it was useful because, in addition to its being a frequent finding in lobular carcinomas, we were able to explain the non-cohesive pattern of the neoplastic cells. In situ and invasive lobular carcinomas characteristically do not express E-cadherin and β-catenin, although the range of aberrant immunoreaction has been reported as between 2.4 and 23.5%, according to several published series [12]. Lobular carcinomas are associated with a higher rate of hormonal receptor-positivity, a low rate of HER-2 expression, and a <14% Ki-67 proliferating index; therefore, the majority of cases are categorized as luminal type-A carcinomas [13]. A 20% Ki-67 proliferating index places our case in the molecular category of luminal type-B carcinoma. Finally, although we were not able to demonstrate the presence of lobular or ductal lesions or any histologic type of invasive carcinoma surrounding the primary tumor, we wonder whether LEL carcinoma of the breast could be considered a distinct immunogenic/molecular variant of invasive lobular or ductal carcinoma, according to the syncytial or dispersed pattern, in addition to the E-cadherin and β-catenin status, or whether it should be considered as a distinct neoplastic entity. Further studies are warranted in order to elucidate the origin and clinic-pathologic features of this singular type of breast carcinoma.
References
- Kumar S, Kumar D. Lymphoepithelioma-like carcinoma of the breast. Mod Pathol. 1994; 7: 129-131.
- Dieci MV, Orvieto E, Dominici M, Conte P, Guarneri V. Rare breast cancer subtypes: histological, molecular, and clinical peculiarities. Oncologist 2014; 19: 805-813.
- Iezzoni JC, Gaffey MJ, Weiss LM. The role of Epstein-Barr virus in lymphoepithelioma-like carcinomas. Am J Clin Pathol. 1995; 103: 308-315.
- Huo Q, Zhang N, Yang Q. Epstein-Barr virus infection and sporadic breast cancer risk: a meta-analysis. PLoS One. 2012; 7: e31656.
- Kulka J, Kovalszky I, Svastics E, Berta M, Füle T. Lymphoepithelioma-like carcinoma of the breast: not Epstein-Barr virus-, but human papilloma virus-positive. Hum Pathol. 2008; 39: 298-301.
- Nio Y, Tsuboi K, Tamaoki M, Tamaoki M, Maruyama R. Lymphoepithelioma-like carcinoma of the breast: a case report with a special analysis of an association with human papilloma virus. Anticancer Res. 2012; 32: 1435-1441.
- Herrera-Goepfert R, Khan NA, Koriyama C, Akiba S, Perez-Sanchez VM. High-risk human papilloma virus in mammary gland carcinomas and non-neoplastic tissues of Mexican women: no evidence supporting a cause and effect relationship. Breast 2011; 20: 184-189.
- Suzuki I, Chakkabat P, Goicochea L, Campassi C, Chumsri S. Lymphoepithelioma-like carcinoma of the breast presenting as breast abscess. World J Clin Oncol. 2014; 5: 1107-1112.
- Cristina S, Boldorini R, Brustia F, Monga G. Lymphoepithelioma-like carcinoma of the breast. An unusual pattern of infiltrating lobular carcinoma. Virchows Arch. 2000; 437: 198-202.
- Pestereli HE, Erdogan O, Kaya R, Karaveli FS. Lymphoepithelioma-like carcinoma of the breast. APMIS. 2002; 110: 447-450.
- Sanati S, Ayala AG, Middleton LP. Lymphoepithelioma-like carcinoma of the breast: report of a case mimicking lymphoma. Ann Diagn Pathol. 2004; 8: 309-315.
- Canas-Marques R, Schnitt SJ. E-cadherin immunohistochemistry in breast pathology: uses and pitfalls. Histopathology. 2016; 68: 57-69.
- Christgen M, Steinemann D, Kühnle E, Länger F, Gluz O, Harbeck N, et al. Lobular breast cancer: Clinical, molecular and morphological characteristics. Pathol Res Pract. 2016; 212: 583-597.