
Case Report
Austin J Clin Case Rep. 2019; 6(3): 1151.
New Insights into Relationship between Nutritional Status and Chemotherapy Tolerance in an Obese Patient
Zheng H1, Pan Q1, You L1, Lou F1, Dong Y1, Niu Z2, Li H1, Wang W1, Sun J1 and Pan H1*
¹Department of Medical Oncology, Sir Run Run Shaw Hospital, College of Medicine, Zhejiang University, Hangzhou, Zhejiang, China
²Department of Radiology, Sir Run Run Shaw Hospital, College of Medicine, Zhejiang University, Hangzhou, Zhejiang, China
*Corresponding author: Hongming Pan, Department of Medical Oncology, Sir Run Run Shaw Hospital, College of Medicine, Zhejiang University, 3#East Qinchun Road, Hangzhou, Zhejiang, China
Received: June 04, 2019; Accepted: July 04, 2019;Published: July 11, 2019
Abstract
We hereby report a case of a 52-year-old Chinese obese male, diagnosed with nasopharyngeal carcinoma. During the first cycle of induction chemotherapy with full weight-based dosing, he developed complication of severe diarrhea, grade III myelosuppression, septic shock, hypovolemic shock and acute kidney injury. He was then transferred to intensive care unit. For this young and treatment-naive patient, in addition to several comorbidities, body composition analysis revealed an abnormality: The patient had normal amount of skeletal muscle mass but excess amount of adipose tissue in viscera and subcutaneous tissue. The patient’s visceral adipose tissue/ subcutaneous adipose tissue ratio was up to 1.10 and was above the mean of average person. As Asians may be fatter than Caucasian in the same BMI, we readdress that the disturbance of fat distribution and fat quantity may also affect the tolerance of chemotherapy drugs and propose new directions that are worth exploring.
Keywords: Chemotherapy toxicity; Obesity; Body composition; Nasopharyngeal carcinoma
Introduction
A number of literatures suggest to incorporate body composition evaluation into chemotherapy dose determination. A large number of these literatures show that low lean body mass or sarcopenia is a significant predictor of toxicity, such as 5-fluorouracil, capecitabine, anthracycline and taxane [1-3]. These studies were mostly finished in Caucasian and few studies have been done in Asian patients. Although Asians may have lower BMI, they have paradoxically higher proportions of body fat compared to Caucasians [4,5]. Wider variations in volumes of visceral adipose tissue than in volumes of skeletal muscle were observed within a cohort of Asian breast cancer patients [6]. This study in Asian breast demonstrates that body fat composition is predictive of doxorubicin-related hematologic toxicities, whereas BSA-based dosing and muscle volume are not. Wejie Gu et al. also reported that radiologic measurement of visceral adipose tissue is an independent prognostic factor for Asian patients treated with targeted therapy for advanced renal cell carcinoma, whereas skeletal muscle is not, which is inconsistent with counterparts in Caucasians [7].
Case Presentation
A 52-year-old Chinese male presented in July 2018 with a painless mass on the left side of the neck, about the size of a pigeon egg. The patient was diagnosed with low/undifferentiated carcinoma of nasopharyngeal stage T3N1M0. In his past history, chronic viral hepatitis B infection was found 20 years ago and treated with entecavir 1# once a day. Hypertension was found over 10 years ago, the highest blood pressure was 180-190/100-110mmHg, and 75mg of irbesartan was taken once a day for hypertension control. Gout was found 3 years ago. Colchicine was taken as needed.
Induction chemotherapy was initiated. The TPF regime (docetaxel + cisplatin + 5-fluorouracil) was adopted in Aug. 8th 2018. Details are shown below: cisplatin 70mg intravenous drip d1, d2+docetaxel 140mg intravenous drip d1+ fluorouracil 3g intravenous injection (pump) 96h. On the day 6, the patient became critically ill. He developed abdominal pain and diarrhea the night before and complained of dry mouth. After 7 or 8 episodes of diarrhea, for watery stool, he became weak and sweaty. The blood pressure measurement was about 80/40mmHg, and the oxygen saturation was reduced to 80%. Laboratory investigations revealed that white cell count, the absolute number of neutrophils and blood platelet count were reduced to 1.5x109/L, 0.87x109/L and 96x109/L respectively. Other abnormal results were: C-reactive protein (CRP) 149.7mg/L, procalcitonin (PCT) 43.73ng/ml. He was treated with recombinant human granulocyte colony stimulating factor 300ug as well as dopamine and fluid infusion. Imipenem/Cilastatin 0.5g intravenous drip q8h was initially applied for the purpose of fighting infection.
At the night of day 6, the patient was in irritable state and sweating profusely and his limbs were wet and cold. The pulse was fast. His vital signs were shown as below: Temperature 38oC, Heartrate 135- 150bpm, Respiration rate 45bpm, Blood pressure 100/60mmHg. He was transferred into intensive care unit (ICU). In ICU, the patient still had watery stools, companying nausea and vomiting. CRP and PCT elevated to 513.4mg/L and >100ng/mL respectively. The patient was diagnosed with septic shock, myelosuppression (blood platelet count dropped to 42x109/L) and acute kidney injury (serum creatinine levels 142μmol/L, BUN 11.47mmol/L). In the day 7, no positive bacteria were detected in fecal culture and PICC catheter culture showed no anaerobic and aerobic bacteria from left and right, Empirical antiinfection treatment was adjusted to: meropenem 1g intravenous drip q8h, teicoplanin 400mg intravenous drip q12h, rifaximin 0.2g oral q6h and metronidazole 400mg oral q8h.On day 11, inflammation markers and blood cell count began to come back to normal and the patient had no complaint of special discomfort, so the patient was transferred back to the general oncology ward.
Second cycle was not initiated until day 35. Drug dose was reduced to 75% in the second cycle: cisplatin 50mg intravenous drip d1, d2+docetaxel 100mg intravenous drip d1+ fluorouracil 2.25g intravenous injection (pump) 96h. After three cycles of induction chemotherapy, therapeutic evaluation was evaluated as complete response (Figure 1).
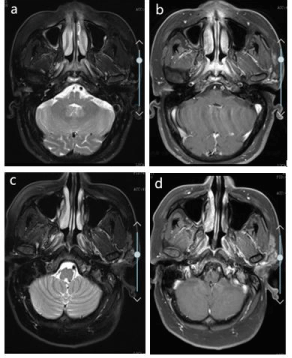
Figure 1: a) MRI T2 scan of nasopharyngeal carcinoma in 2018-08-07; b)
MRI T1 +C scan of nasopharyngeal carcinoma in 2018-08-07; c) MRI T2
scan of same region in 2018-10-08; d) MRI T1 +C scan of nasopharyngeal
carcinoma in 2018-10-08.
Discussion
The toxicity of cancer patients receiving chemotherapy is difficult to predict and the large inter-individual variability in drug exposure exists. Considering only from the patients’ condition, many factors can influence tolerance to a given drug, which include patient characteristics (e.g., age, performance status, ethnicity, weight), physiological factors (e.g., comorbidities, liver and renal function, protein levels) and intrinsic factors (e.g., genetic variations) [8].
In light of the severe diarrhea in this patient, we also tested the genetic status of dihydropyridine dehydrogenase (DPYD) enzyme. DPYD enzyme deficiency leads to toxic accumulation of fluorouracil metabolites in vivo, but the patient’s was DPYD (CC) wild-type.
This patient, though young and undergoing chemotherapy for the first time, experienced such deadly side effect, the cause of which is worth exploring. The patient’s comorbidities (chronic viral hepatitis B, hypertension and gout) may be part of explanation. According to the CT before chemotherapy, the patient has fatty liver disease. Laboratory tests also showed slight increases in AST and ALT before induction chemotherapy. These lesions in the liver can possibly influence liver blood flow and indirectly affect hepatic metabolism. In this induction chemotherapy regime, docetaxel and fluorouracil are mainly metabolized by the liver. Docetaxel was metabolized by CYP3A4 isoenzyme and the activity of cytochrome P4503A4 in obese individuals was proven to decrease [9].
It is reasonable to attribute the severe side effect to the patient’s background condition. What is more noteworthy would be the patient’s disorders of body composition. The patient is 174cm in height, 95kg in weight. His body mass index is 31.4kg/m2 and body surface area is 2.14m2, (calculated using the Mosteller formula: BSA (m2) = ([height (cm) × weight (kg)]/3600)1/2. This patient’s ideal body weight and ideal body surface area are 66.6kg and 1.79m2 (calculated using this formula: ideal body weight (kg) = height (m)2×22) [10]. For further analysis of his body composition, we utilized the CT image before the initiation of induction chemotherapy using Slice-OMatic software (v5.0Tomovision, Montreal, Canada).With standard operating procedures, skeletal muscle mass (SMM), skeletal muscle density (SMD), visceral adipose tissue (VAT), subcutaneous adipose tissue (SAT) and inter-muscular adipose tissue (IMAT) were assessed using this software on axial slices at the third lumbar vertebra (L3) (Figure 2). Skeletal muscle index (SMI = SMM/height2) 64.28cm2/ m2, SMD 44.24HU, VAT 196.80cm2, SAT 179.2cm2, IMAT 17.69cm2. This patient has a relatively normal or high skeletal muscle index, compared to the mean SMI of all Caucasian objects in Aaron J. Grossberg’s. In his study, all male objects with head and neck squamous cell carcinoma have a mean SMI of 56.4±9.5 cm2/m2 before radiotherapy [11].
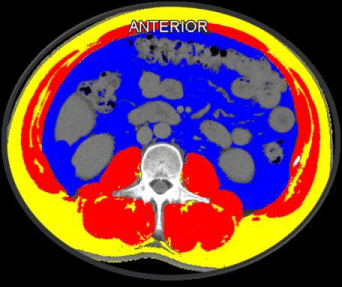
Figure 2: Axial CT image of the third lumbar vertebral region with
corresponding highlighted body composition in patients: skeletal muscle
mass (SMM) in red, visceral (VAT) in blue, subcutaneous fat tissues (SAT) in
yellow, and muscle fat infiltration (IMAT) in gree.
All the time, obesity has been recognized by BMI. However, patients with the same BMI may have huge differences in body compositions. Sarcopenic obesity, a condition of combined sarcopenia and obesity, has been associated with negative clinical outcomes including dose-limiting toxicity [13]. But for this musclerich patient, how does body composition affect drug toxicity? So, we’re looking for other indicators of body composition that might explain this toxic side effect.
As we learn from this case, the patient has an excess of adipose tissue in viscera and subcutaneous tissue. Adipose tissue has been increasingly known as a complex secretory organ that secrets proinflammatory cytokines (interleukin-6, interleukin-1, and tumour necrosis factor-a), which could contribute to the development of cancer [14]. Moreover, visceral adipose tissue has been associated with a greater degree of obesity-related metabolic derangements than subcutaneous fat [14,15]. In vivo, data has shown that subcutaneous fat cells can exert a metabolically advantageous function [16], which means subcutaneous fat-dominant obesity may be a “metabolically healthy” status. That explains the rationale for evaluating VAT/SAT ratio [17]. VAT/SAT ratio has been an emerging obesity index in many clinical studies, examined in positive relation with an increased risk of colorectal adenoma (mean VAT/SAT ratio in adenoma group and no adenoma group: 1.09±0.60 vs. 0.94±0.82, p‹0.001), shorter disease-free survival of colorectal cancer (mean VAT/SAT ratio in all subjects: 0.83±0.42) and severity of coronary artery disease (mean VAT/SAT ratio in risk group: 0.95±0.33 vs.0.70±0.25, p‹0.001) [18-20]. Until now, there is no standard classification of VAT/SAT ratio. The patient’s ratio is up to 1.10 and is above the mean of the risk group in above-mentioned studies. His VAT and SAT value are also more than the mean value of above-mentioned studies. So far, little research has been done on the relationship between VAT/ SAT and side effect of chemotherapy. It provides a new direction for further clinical trials to prospectively test relationships between body composition and chemotherapy toxicity.
Conclusion
The analysis of comorbidities, nutritional status and body composition presented by visceral adipose tissue/subcutaneous adipose tissue ratio that affect metabolism should be paid higher attention for the sake of personalized treatment. The variability of cut offs in sarcopenia, visceral adipose tissue/ subcutaneous adipose tissue ratio can be explained by ethnicity, life style, cancer type and disease stage. As Asians may have higher proportions of fat and fewer proportions of skeletal muscle than Caucasians under the same condition, the cut offs of sarcopenia proposed by the international consensus of cancer cachexia may not be suitable for Asians. And when analyzing body composition, besides skeletal muscle area, fat distribution and quantity also deserve further attention, especially in Asians.
Acknowledgement
This work was supported by Zhejiang provincial medicine and health discipline platform project (clinical research) 2018ZD029.
Statement of Ethics
Our patient has given written informed consent to publish their case (including publication of images). All procedures performed in this case report involving human participants were in accordance with the ethical standards of the institutional and national research comi1964 Helsinki declaration and its later amendments or comparable ethical standards.
References
- Prado CM, Baracos VE, McCargar LJ, Reiman T, Mourtzakis M, Tonkin K, et al. Sarcopenia as a determinant of chemotherapy toxicity and time to tumor progression in metastatic breast cancer patients receiving capecitabine treatment. Clin Cancer Res. 2009; 15: 2920-2926.
- Prado CM, Baracos VE, McCargar LJ, Mourtzakis M, Mulder KE, Reiman T, et al. Body composition as an independent determinant of 5-fluorouracilbased chemotherapy toxicity. Clin Cancer Res. 2007; 13: 3264-3268.
- Shachar SS, Deal AM, Weinberg M, Williams GR, Nyrop KA, Popuri K, et al. Body Composition as a Predictor of Toxicity in Patients Receiving Anthracycline and Taxane-Based Chemotherapy for Early-Stage Breast Cancer. Clin Cancer Res. 2017; 23: 3537-3543.
- Deurenberg-Yap M, Schmidt G, van Staveren WA, Deurenberg P. The paradox of low body mass index and high body fat percentage among Chinese, Malays and Indians in Singapore. International journal of obesity and related metabolic disorders: journal of the International Association for the Study of Obesity. 2000; 24: 1011-1017.
- Wang J, Thornton JC, Russell M, Burastero S, Heymsfield S, Pierson RN Jr. Asians have lower body mass index (BMI) but higher percent body fat than do whites: comparisons of anthropometric measurements. The American journal of clinical nutrition. 1994; 60: 23-28.
- Wong AL, Seng KY, Ong EM, Wang LZ, Oscar H, Cordero MT, et al. Body fat composition impacts the hematologic toxicities and pharmacokinetics of doxorubicin in Asian breast cancer patients. Breast Cancer Res Treat. 2014; 144: 143-152.
- Gu W, Zhu Y, Wang H, Zhang H, Shi G, Liu X, et al. Prognostic value of components of body composition in patients treated with targeted therapy for advanced renal cell carcinoma: a retrospective case series. PLoS One. 2015; 10: e0118022.
- Hempel G, Boos J. Flat-fixed dosing versus body surface area based dosing of anticancer drugs: there is a difference. The oncologist. 2007; 12: 913-923.
- Kotlyar M, Carson SW. Effects of obesity on the cytochrome P450 enzyme system. International journal of clinical pharmacology and therapeutics. 1999; 37: 8-19.
- Miyahara T, Mochinaga S, Kimura S, Aragane N, Yakabe T, Morita S, et al. Effects of tumor type, degree of obesity, and chemotherapy regimen on chemotherapy dose intensity in obese cancer patients. Cancer Chemother Pharmacol. 2013; 71: 175-182.
- Grossberg AJ, Chamchod S, Fuller CD, Mohamed AS, Heukelom J, Eichelberger H, et al. Association of Body Composition With Survival and Locoregional Control of Radiotherapy-Treated Head and Neck Squamous Cell Carcinoma. JAMA oncology. 2016; 2: 782-789.
- Li Z, Bowerman S, Heber D. Health ramifications of the obesity epidemic. Surg Clin North Am. 2005; 85: 681-701.
- Carneiro IP, Mazurak VC, Prado CM. Clinical Implications of Sarcopenic Obesity in Cancer. Current oncology reports. 2016; 18: 62.
- van Kruijsdijk RC, van der Wall E, Visseren FL. Obesity and cancer: the role of dysfunctional adipose tissue. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2009; 18: 2569-2578.
- Ibrahim MM. Subcutaneous and visceral adipose tissue: structural and functional differences. Obesity reviews: an official journal of the International Association for the Study of Obesity. 2010; 11: 11-18.
- Tran TT, Yamamoto Y, Gesta S, Kahn CR. Beneficial effects of subcutaneous fat transplantation on metabolism. Cell metabolism. 2008; 7: 410-420.
- Fujiwara N, Nakagawa H, Kudo Y, Tateishi R, Taguri M, Watadani T, et al. Sarcopenia, intramuscular fat deposition, and visceral adiposity independently predict the outcomes of hepatocellular carcinoma. Journal of hepatology. 2015; 63: 131-140.
- Nagata N, Sakamoto K, Arai T, Niikura R, Shimbo T, Shinozaki M, et al. Visceral abdominal fat measured by computed tomography is associated with an increased risk of colorectal adenoma. Int J Cancer. 2014; 135: 2273-2281.
- Gao Y, Wang YC, Lu CQ, Zeng C, Chang D, Ju S. Correlations between the abdominal fat-related parameters and severity of coronary artery disease assessed by computed tomography. Quantitative imaging in medicine and surgery. 2018; 8: 579-587.
- Moon HG, Ju YT, Jeong CY, Jung EJ, Lee YJ, Hong SC, et al. Visceral obesity may affect oncologic outcome in patients with colorectal cancer. Annals of surgical oncology. 2008; 15: 1918-1922.