
Case Report
Austin J Clin Case Rep. 2020; 7(1): 1162.
Ancylostoma ceylanicum First ever Case Detected in Sri Lanka
Mallawarachchi CH* and Samarasinghe S
Medical Research Institute, Sri Lanka
*Corresponding author: Mallawarachchi CH, Medical Research Institute, P.O. Box 527, Dr Danister De Silva Mawatha (Baseline road), Colombo 08, 00800, Sri Lanka
Received: April 23, 2020; Accepted: June 23, 2020; Published: June 30, 2020
Abstract
Background: Hook worm infection is a soil transmitted helminthiasis (STH) which is acquired by human with intradermal penetration of filariform larvae. Necator americanus is the recognized causative agent in Sri Lanka and it’s prevalence 0.29%. Ancylostoma ceylanicum is a recognized emerging zoonotic hook worm infection in Southeast Asian region and West Pacific Ocean regions. Canines and felines act as reservoir hosts. Cat and dog population is very high in Sri Lanka and they are the reservoir hosts for this infection.
Case presentation: A 70 years female was admitted to the hospital with epigastric pain and abdominal discomfort. After admission upper gastro intestinal (GI) endoscopy was performed and a live worm was extracted from the duodenum. Following that patient was treated with 400mg single dose of Albendazole and it was repeated after two weeks. Her clinical symptoms has subsided with the treatment. The worm has been sent to the department of Parasitology, Medical Research Institute (MRI), Sri Lanka for identification. After through observation and examination it was confirmed as Ancylostoma ceylanicum hook worm. This is the first time A. ceylanicum worm was found from a human in Sri Lanka.
Conclusion: The emerging and remerging zoonotic infections are increasing in Sri Lanka as a result of high dog and cat population and their close contact with humans.
Keywords: Hook worm; Ancylostoma ceylanicum; Sri Lanka; Dogs; Zoonotic
Abbreviations
STH: Soil Transmitted Helminthiasis; GI: Gastro Intestinal; MRI: Medical Research Institute; CLM: Cutaneous Larvae Migrans
Introduction
The hookworm infection which is acquired by the penetration of human skin by Ancylostoma duodenale and Necator americanus [1]. N. americanus is the recognized causative agent of hook worm infection in Sri Lanka and its prevalence is found to be 0.29% locally [10]. Other than these two anthropophilic species, hook worm of cats and dogs such as Ancylostoma ceylanicum, Ancylostoma braziliense, Ancylostoma caninum can cause zoonotic human infections [2]. A. braziliense and A. caninum can cause cutaneous larvae migrans (CLM) while A. ceylanicum is capable of causing patent infection in humans [3-5]. Recent molecular studies have shown that A. ceylanicum is the second most prevalent hookworm species in humans’ in the South East Asia [6-8].
Case Presentation
A 70 years old female patient from Veyangoda, Sri Lanka has gone to a base hospital for epigastric pain and abdominal discomfort. An upper GI endoscopy was performed, and a live worm has been observed in the duodenum. The worm was extracted and sent to the department of Parasitology, MRI, Sri Lanka for identification.
This patient is married with four grown up children who lived separately. She had not gone outside the country but one of her daughters was a foreign employer for 14 years and she visits Sri Lanka on and off. The patient usually walks to close by lands bare footed to collect firewood, cow dung etc.
She uses a water sealed latrine. She does not have cats and dogs at home, but stray dogs and cats were seen roaming around the house.
She had not taken anthelminthic treatment for a time of five years by the time she got these symptoms. Following the endoscopy, she had given Albendazole 400mg single dose. The same dose was repeated after two weeks. Her clinical symptoms have subsided with this treatment. Six weeks after the second dose, she was given Albendazole again for Pruritis ani.
Stool samples were collected from the patient, her family and neighbours to examine for helminth ova using Kato-Katz technique and all were found negative. The patient’s stool sample was taken only after giving the third dose of Albendazole. The ward staffs were so keen to treat patient and they have forgotten to take a stool before giving the treatment to the patient.
This patient had undergone several laboratory investigations before the upper GI endoscopy and her full blood count was normal except for a mild has anaemia (Hb – 11.4g/dl). Her stools were negative for occult blood and nothing significant was found from the other investigation as well.
Description of the worm
The worm was a female worm. The total length of the worm was 9mm and the width was 48μm at the middle. There was a mucron at the posterior end. It had a stumpy appearance and length was 7 μm. The cuticle width was 12μm. At the buccal cavity two teeth were clearly visible in one side and the other side teeth were present but twisted medially. The lateral teeth were larger than the medial ones and it would be seen clearly on the left side (Figure 1). The width of the transverse striations which was present was 5μm. There was a sharp ventral bend posterior to the vulva.
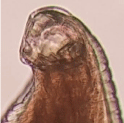
Figure 1: Buccal cavity.
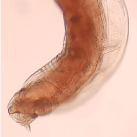
Figure 2: Posterior end with mucron.
Discussion
The hookworm infection is a Soil-transmitted helminthiasis (STH) and transmitted by intradermal penetration of infective larvae. N. americanus and A. duodenale are generally considered as the most common species of human hookworm disease [9]. A. duodenale is not reported in Sri Lanka. The hook worm prevalence is 0.29% in school children aged 5-7 years in 2017 [10].
The CLM due to hookworms of dogs and cats, such as A. caninum and A. braziliense are a known zoonotic infection in Sri Lanka [11]. Their adult forms are not reported in humans [3]. A high prevalence of CLM reported (58.2%), among the devotees performing “side roll” during the festival at Nallur Temple, Jaffna, Sri Lanka [11]. A case of eye infection due to A. tubaeforme was reported by A.S. Dissanaike in 2000 [12]. A. ceylanicum is recognised as an emerging public health risk in some communities and adults are identified in humans in some Southeast Asian region and West Pacific Ocean region countries [13-16].
The prevalence of A. ceylanicum hookworms in cats and dogs ranges from 24% to 92% in the Asia-Pacific region [6,17-19]. In Sri Lanka, a high prevalence of Ancylostoma infection (73.3 %) has been identified among dog population in Kandy district by Perera et al in 2012 but they have not speciated that [20].
The specimen, we described here could be identified as a worm which belong to Ancylostoma spp due to teeth in the buccal cavity instead of cutting plates which is specific for N. americanus. Presence of a mucron at the posterior end in spite of expanded copulatory bursa indicates that this is a female worm.
A. tubaeforme and A. caninum have three teeth on either side of the buccal capsule [21]. As this worm has only two teeth on either side, it is definite that this worm does not belong to above two species. A. duodenale, A. braziliense and A. ceylanicum have two teeth either side of the buccal capsule [22,23]. The mucron of the A. duodenale is a long ( about 21μm) and slender [24]. The mucron of this worm is shorter (5μm) and stout. This feature excluded the possibility of this worm being A. duodenale.
A. braziliense and A. ceylanicum could be differentiated from the distance between the transverse striations and observing the margin of the buccal capsule [20,25,26]. The distance between transverse striations of A. braziliense is 8-9 μm and it is 4-5 μm in A. ceylanicum [22]. In our specimen distance between transverse stration was 5μm. This feature favours the worm to be A. ceylanicum. At the margin of the buccal capsule there is a small tubercular process in A. braziliense [20]. In this case that process is absent. when, all these features are considered we can confirm this is a female A. ceylanicum.
Sri Lanka is a country, where there is a high cat and dog population. A recent survey has revealed the dog: human population ratio is 1:8.5 [27]. An upward trend of emerging and re-emerging zoonotic infections related to canine and feline population has been shown in the country [28,29]. Although, this is the first time an adult A. ceylanicum is detected in a human, there may be similar cases which go unnoticed due to lack of suspicion. Even in this case the same day they had extracted the worm, anthelminthic treatment was given to the patient before taking a stool sample from the patient to look for Ancylostoma ova. There is no wonder that patient’s stool samples was negative for helminth ova as we got opportunity to examine the stool samples from the patient only after the helminthic treatment is given.
STH prevalence is very low in Sri Lanka [10] though poor sanitation and the lack of use of footwear favour transmission of hookworm infection in lower socioeconomic classes. This community is practising good sanitary disposal of human feaces and barefooted people are rare.
Due to high population of dogs and cats in human habitation, contamination of soil with animal stool is unavoidable. The people who work outdoor barefooted are invariably at a risk of getting zoonotic hookworm infections. The stool samples tested from the patient’s household and neighbourhood were also negative. That may be due to wearing of footwear and low transmission potential of zoonotic infections.
Conclusion
Emerging and re-emerging zoonotic infections related to dogs and cats are increasing in Sri Lanka due to high dog and cat population together with their close contact with humans. The possibility of A. ceylanicum as a cause of potential zoonotic infections in Sri Lanka should not be disregarded.
Acknowledgements
Authors are grateful to acknowledge the support given by Mrs. Thushara Thanthirigae for laboratory works.
References
- Chan MS, Medley GF, Jamison D, Bundy DA. The evaluation of potential global morbidity attributable to intestinal nematode infections. Parasitology. 1994; 109: 373–387.
- Bowman DD, Montgomery SP, Zajac AM, Eberhard ML, Kazacos KR. Hookworms of dogs and cats as agents of cutaneous larva migrans. Trends Parasitol. 2010; 26: 162–167.
- Kim T-H, Lee B-S, Sohn W-M. Three clinical cases of cutaneous larva migrans. Korean J Parasitol. 2006; 44: 145.
- Yoshida Y, Okamoto K, Chiu JK. Ancylostoma ceylanicum infection in dogs, cats, and man in Taiwan. Am J Trop Med Hyg. 1968; 17: 378–381.
- Carroll SM, Grove DI. Experimental infection of humans with Ancylostoma ceylanicum: clinical, parasitological, haematological and immunological findings. Trop Geogr Med. 1986; 38: 38–45.
- Ngui R, Lim YAL, Traub R, Mahmud R, Mistam MS. Epidemiological and genetic data supporting the transmission of Ancylostoma ceylanicum among human and domestic animals. PLoS Negl Trop Dis. 2012; 6: e1522.
- Traub RJ, Inpankaew T, Sutthikornchai C, Sukthana Y, Thompson RCA. PCR-based coprodiagnostic tools reveal dogs as reservoirs of zoonotic ancylostomiasis caused by Ancylostoma ceylanicum in temple communities in Bangkok. Vet Parasitol. 2008; 155: 67–73.
- Inpankaew T, Schär F, Dalsgaard A, Khieu V, Chimnoi W, Chhoun C, et al. High Prevalence of Ancylostoma ceylanicum Hookworm Infections in Humans, Cambodia. 2012. Emerg Infect Dis. 2014; 20.
- de Silva NR, Brooker S, Hotez PJ, Montresor A, Engels D, Savioli L. Soiltransmitted helminth infections: updating the global picture. Trends Parasitol. 2003; 19: 547–551.
- de Silva NR, Gunawardane K, Gunawardane S, Iddawela D, Kanathasan S, Muruganathan A, et al. National Survey of Intestinal Nematode Infection in Sri Lanka. University of Kelaniya, Dalugama, Kelaniya, Sri Lanka. 2017.
- Kannathasan S, Murugananthan A, Rajeshkannan N, de Silva NR. Cutaneous Larva Migrans among Devotees of the Nallur Temple in Jaffna, Sri Lanka. Stoute JA, editor. PLoS ONE. 2012; 7: e30516.
- Dissanaike AS, Ihalamulla RL, De Silva D, Pathirana S, Weerakoon U, Amaratunga MS, et al. On a dead female hookworm, probably Ancylostoma tubaeforme, from the vitreous of a patient in Sri Lanka. Ceylon J Med Sci. 2000; 43: 25.
- Lim KN, Ismail WHW, Lim YAL, Mahmud R, Ngui R. Zoonotic Ancylostoma ceylanicum Infection Detected by Endoscopy. Am J Trop Med Hyg. 2014; 91: 86–88.
- Speare R, Bradbury RS, Croese J. A Case of Ancylostoma ceylanicum Infection Occurring in an Australian Soldier Returned from Solomon Islands. Korean J Parasitol. 2016; 54: 533–536.
- Yoshikawa M, Ouji Y, Hirai N, Nakamura-Uchiyama F, Yamada M, Arizono N, et al. Ancylostoma ceylanicum, novel etiological agent for traveler’s diarrhea—report of four Japanese patients who returned from Southeast Asia and Papua New Guinea. Trop Med Health. 2018; 46.
- Smout FA, Skerratt LF, Butler JRA, Johnson CN, Congdon BC, Thompson RCA. The hookworm Ancylostoma ceylanicum: An emerging public health risk in Australian tropical rainforests and Indigenous communities. One Health Amst Neth. 2017; 3: 66–69.
- Traub RJ. Ancylostoma ceylanicum, a re-emerging but neglected parasitic zoonosis. Int J Parasitol. 2013; 43: 1009–1015.
- Scholz T, Uhlírová M, Ditrich O. Helminth parasites of cats from the Vientiane Province, Laos, as indicators of the occurrence of causative agents of human parasitoses. Parasite. 2003; 10: 343–350.
- Traub RJ, Inpankaew T, Sutthikornchai C, Sukthana Y, Thompson RCA. PCR-based coprodiagnostic tools reveal dogs as reservoirs of zoonotic ancylostomiasis caused by Ancylostoma ceylanicum in temple communities in Bangkok. Vet Parasitol. 2008; 155: 67–73.
- Perera P, Rajapakse R, Rajakaruna R. Gastrointestinal parasites of dogs in Hantana area in the Kandy District. J Natl Sci Found Sri Lanka. 2013; 41: 81.
- Burrows RB. (Comparative Morphology of Ancylostoma tubaeforme (Zeder, 1800) and Ancylostoma caninum (Ercolani, 1859). J Parasitol. 1962; 48: 715–718.
- Garcia LS. Diagnostic medical parasitology. 5th ed. Washington, D.C: ASM Press. 2007: 1202.
- Yoshida Y. Comparative Studies on Ancylostoma braziliense and Ancylostoma ceylanicum. I. The Adult Stage. J Parasitol. 1971; 57: 983–989.
- Yoshida Y, Matsuo K, Kondo K, Arizono N, Ogino K. Scanning electron microscopy of hookworms. 2. Adults and infective-stage larvae of Ancylostoma duodenale (Dubini, 1843). Southeast Asian J Trop Med Public Health. 1974; 5: 515–518.
- Bowman DD, editor. Feline clinical parasitology. 1st ed. Ames: Iowa State University Press; 2002. 469 p.
- Biocca E. On Ancylostoma braziliense (de Faria, 1910) and its Morphological Differentiation from A. ceylanicum. J Helminthol. 1951; 25: 1.
- Pimburage RMS, Harischandra PAL, Gunatilake M, Jayasinhe DN, Balasuriya A, Amunugama RMSK. A cross-sectional survey on dog ecology and dog anti-rabies vaccination coverage in selected areas in Sri Lanka. Sri Lanka Vet J. 2017; 64: 1.
- Dissanaike AS, Abeyewickreme W, Wijesundera MD, Weerasooriya MV, Ismail MM. Human dirofilariasis caused by Dirofilaria (Nochtiella) repens in Sri Lanka. Parassitologia. 1997; 39: 375–382.
- Mallawarachchi CH, Nilmini Chandrasena TGA, Premaratna R, Mallawarachchi SMNSM, de Silva NR. Human infection with sub-periodic Brugia spp. in Gampaha District, Sri Lanka: a threat to filariasis elimination status? Parasit Vectors. 2018; 11.