
Case Report
Austin J Clin Case Rep. 2020; 7(2): 1168.
Multiplex PCR for Cryptococcal Meningoencephalitis - Calling for Caution
Ravi Bhushan1, Neha Gupta1*, Atmaram Bansal2, Arun Garg2, Jayesh Modi2, Rajiv Gupta3, Reeshika Verma3, Dinesh Bansal3, Vijay Kher4, Rashmi4, Teena Wadhwa5, Smita Sharma5 and Rajeev Soman6
1Division of Infectious Diseases, MEDANTA- The Medicity, India
2Department of Neurology, MEDANTA- The Medicity, India
3Department of Radiology, MEDANTA- The Medicity, India
4Institute of Nephrology, MEDANTA- The Medicity, India
5Department of Microbiology, Medanta- The Medicity, Gurugram, Haryana, India
6Consultant Infectious Diseases, Jupiter Hospital, Pune, India
*Corresponding author: Neha Gupta, Consultant-Infectious Diseases, MEDANTA- The Medicity, Gurgaon, India
Received: July 16, 2020; Accepted: August 21, 2020; Published: August 28, 2020
Abstract
Worldwide cryptococcal meningo encephalitis is associated with prolonged antifungal therapy, significant morbidity and mortality. Newer molecular assays are being developed for a rapid diagnosis. We present two cases of culture positive cryptococcal meningo encephalitis where the molecular PCR panel failed to detect the Cryptoccoccus resulting in delay in diagnosis. Caution and careful interpretation of such molecular platforms and multiple testing methods are advised in suspected cryptococcal meningitis.
Keywords: Cryptococcal Meningitis, BioFire Film Array Meningitis/Encephalitis Panel; Molecular Diagnosis, Cryptococcal Meningo Encephalitis; Cryptococcal Ag Test
Introduction
Cryptococcosis is a fungal infection caused by Cryptococcus neoformans and Cryptococcus gattii and accounts for the most common cause of fungal meningitis worldwide [1]. The range of clinical infections includes chronic skin infections, lung infections and meningo encephalitis. Delay in diagnosis of cryptococcal meningitis is associated with increased morbidity and mortality [2,3]. Diagnostic modalities include Cryptococcal Antigen (CrAg) detection tests, culture and the Biofire Meningitis/Encephalitis (ME) panel (bioMerieux, Marcy l’Etoile, France). Culture remains the gold standard for diagnosis of cryptococcal disease [4] but it is time consuming and labor intensive. Cryptococcal Antigen (CrAg) lateral flow assays are rapid, specific, and may be more sensitive than culture when the burden of organism is low [4]. However, in patients presenting with symptoms of meningo encephalitis, the causative organism is frequently not clinically apparent. There can be a large potential differential diagnosis requiring consideration, and there may be limited available Cerebral Spinal Fluid (CSF) for testing. Efforts have been made in recent years to develop assays that would allow testing for a wide variety of pathogens associated with meningo encephalitis in a short time frame and using small volumes of CSF. The Film Array Meningitis/Encephalitis (ME) Panel (BioFire Diagnostics, Salt Lake City, UT) uses multiplex Polymerase Chain Reaction (PCR) to test for 14 targets using just 200 μL of CSF with a reported hands-on time of 2 minutes and a turnaround time of approximately 1 hour [5]. The fast turnaround time allows clinicians to rapidly initiate targeted therapies. However, as with all diagnostic tools, clinical correlation is of paramount importance and clinicians need to be cautious in interpreting results from such PCR panels. Herein we report two cases of cryptococcal meningoencephalitis with false-negative results with the Biofire meningitis/encephalitis panel.
Case 1
A 50-year gentleman sought medical attention in 2018 for headache of 5 days duration associated with weakness and fever with chills for the past 2-3 days. Headache was severe in intensity, diffuse, non-radiating and was not associated with nausea, vomiting, seizure, loss of consciousness or focal neurological deficit. He had undergone a live-donor kidney transplant 5 years ago. He had subsequently developed chronic Antibody Mediated Rejection (AMR) with a baseline creatinine 2.3 mg/dl and was receiving tacrolimus, azathioprine and prednisolone as immunosuppressive therapy.
On examination, patient was conscious, well oriented to time, place, and person; vitals were stable, afebrile. On systemic examination, no abnormalities were detected. On neurological examination, neck rigidity was present; higher mental functions were normal with no cranial nerve abnormalities. Patient had no motor deficit and normal fundus examination.
His laboratory evaluation showed Hemoglobin (Hb) 9.1 mg/dl, Total Leucocyte Count (TLC) was 10590 cells/cm3 with platelets 1.93 lakh/cumm, creatinine was 2.5 mg/dl, and Liver function tests were normal. Blood and urine cultures were sterile. Non-Contrast Computerized Tomography (NCCT) brain revealed a few small focal hypodensities in supratentorial periventricular and subcortical white matter suggestive of chronic microvascular ischaemic changes. Rest of the brain parenchyma revealed normal attenuation. Magnetic Resonance Imaging (MRI) brain findings are suggestive of changes of Fazekas grade I to II chronic microvascular ischemic changes in supratentorial white matter. No acute infarct/intra-parenchymal or subdural hematoma/venous sinus thrombosis or intracranial space-occupying mass lesion is seen. No evidence of PRES/osmotic demyelination is seen. NCCT chest reveals few conglomerate nodular opacities seen in left upper lobe anterior segment with a possible infective etiology.
CSF analysis revealed Total Cell Count (TCC) 284 cells/cm3 (41% neutrophils, 58% lymphocytes), glucose of 78 mg/dl, and protein of 362 mg/dl, ADA- 3.90 and a negative Xpert MTB/RIF test. India-Ink was negative; no fungal element seen on KOH mount; no acid fast bacilli seen on ZN staining; No pus cells and micro-organism seen on Gram staining; No acid fast branching filamentous bacilli morphologically resembling Nocardia seen on modified ZN staining. CMV DNA was not detected. No organism was detected by Rapid meningitis PCR (film array) panel (included E.coli K1, H. influenzea, Listeria monocytogenes, Neisseria meningitides, streptococcus agalactiae, Streptococcus pneumoniae, Cytomegalovirus, Enterovirus, HSV1, HSV2, HHV6, Human parechovirus, Varicella zoster virus, Cryptococcus neoformans/gatti). Cryptococcal Antigen (CrAg) was detected in CSF by immune-chromatography test (lateral flow assay). CSF aerobic and anaerobic cultures were sterile. CSF fungal culture grew Cryptococcus neoformans after 5 days of incubation. Infectious Diseases (ID) consultation was sought by the primary team and patient was initiated on induction therapy with liposomal amphotericin B (L-AmB) and Flucytosine (5-FC).
Case 2
A 40-year old gentleman with well controlled T2DM on oral hypoglycemic agent presented in January 2020 with fever, headache, multiple episodes of vomiting since 1 month and neck pain since last 2 days. Fever was intermittent. Headache was frontal, dull aching, non-radiating, mild to moderate in severity, increased when turning towards right and flexion of head. Patient is a resident of Delhi NCR and had a recent travel history to the urban areas in Uttar Pradesh and Gujarat. There was no history of skin rash or abscesses, injury or high-risk behavior. During illness, patient had consulted local doctors and was treated with antibiotics with no significant improvement.
On examination, patient was conscious, well oriented to time, place and person; vitals were stable, afebrile. On systemic examination, no abnormalities were detected. On neurological examination, neck rigidity was present; higher mental functions were normal with no cranial nerve abnormalities. Patient had no motor deficit, power- 5/5 in all limbs, extensor planter response, normal fundus examination.
Laboratory reports revealed TLC 11,100 cells/cm3 with normal CRP, ESR (7mm/hour), kidney function test, and HbA1c-6.3%.
Contrast MRI brain screening revealed multifocal areas of signal alteration and enhancement in supratentorial brain parenchyma, brain stem and cerebellum. The imaging findings were reported to be suggestive of infective etiology with possibility non-specific viral encephalitis/cerebellitis (Figure 1).
MRI spine contrast cervical revealed mild degenerative spondylotic changes; abnormal signal and enhancement in bilateral cerebellum; no significant spinal stenosis, cord compression; no abnormal leptomeningeal or intramedullary enhancement; no significant focal intramedullary lesion detected.
Lumber puncture was performed and the CSF analysis revealed a TCC of 77/µl with 99% of lymphocytes, glucose- 88mg/dl, protein- 70mg/dl; no organism seen on Grams stain; no fungal element seen on KOH mount; no acid fast bacilli seen on ZN stain; India Ink was negative for encapsulated budding yeast cells. Mycobacterium TB was not detected by Xpert MTB/RIF test; No organism was detected by Rapid meningitis PCR (film array) panel (included E.coli K1, H. influenzea, Listeria monocytogenes, Neisseria meningitides, streptococcus agalactiae, Streptococcus pneumoniae, Cytomegalovirus, Enterovirus, HSV1, HSV2, HHV6, Human parechovirus, Varicella zoster virus, Cryptococcus neoformans/gatti. CSF CrAg was positive by immunochromatography test (Figure 2).
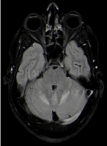
Figure 1: MRI brain suggestive of multifocal areas of signal alteration and enhancement in supratentorial brain parenchyma, brainstem and cerebellum.
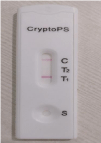
Figure 2: Cryptococcal Ag was detected in CSF by immunochromatography test (lateral flow assay).
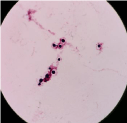
Figure 3: CSF containing bottle flashed positive which showed budding yeast cells on Gram stain.
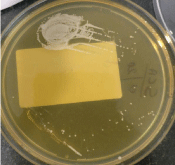
Figure 4: Growth of creamy white colonies of Cryptococcus on SCA media.

Figure 5: Identification and anti-fungal susceptibility report after prolonged incubation of CSF.
ID consultation was sought by the neurology team. Based on the history, imaging, and laboratory reports with a positive CrAg- a provisional diagnosis of cryptococcal meningo encephalitis was considered. Patient was started on L-AmB and 5-FC. The microbiology lab was requested to keep the CSF cultures for prolonged incubation of 15 days. Patient started improving on L-AmB and 5-FC. On 7th day of aerobic culture, bottle flashed positive which showed budding yeast like cells on Gram stain (Figure 3) and grew on Sabouraud chloramphenicol agar (Figure 4). Identification and anti-fungal susceptibility was done by VITEK 2 Compact (bioMeriux) which showed Cryptococcal neoformans (sensitive to AmB, 5-FC, voriconazole, fluconazole) (Figure 5).
Discussion
These cases highlight certain important issues associated with the use of PCR panels such as the Film Array ME Panel in clinical practice. We describe one non-human immunodeficiency virus infected, non-transplant patient and one renal transplant recipient with cryptococcal meningitis with positive CrAg and Cryptococcus culture that was not detected on the Biofire ME panel.
Fungal cultures were performed on brain heart-infusion agar (370 C) and Sabouraud dextrose agar (300 C) incubated in ambient air. Identification was initially performed by VITEK 2 Compact (bioMerieux, Marcy l’Etoile, France).
The reason for the false-negative results was thought to be due to low fungal counts below the lower limit of detection of the Biofire ME panel. According to the kit insert, the limit of detection of the assay was 100 CFU/mL for both Cryptococcus spp. On literature review, we found that the clinical assay performance of the Film Array ME Panel was assessed in a multicenter study that examined 1560 residual CSF samples from patients undergoing lumbar puncture at 11 sites across the United States. In this study, the results from the Film Array ME Panel were compared with comparator. The comparator was CSF culture for the bacteria and bidirectional PCR sequencing for the viruses and fungi included in the panel. The sensitivity for Cryptococcus neoformans/gattii was reported at 100% (1/1) and was included in the overall assay sensitivity despite being calculated from a single positive specimen. Specificity for Cryptococcus spp. was reported as 99.7% (1555/1559). Of note, the comparator method for Cryptococcus in this study was PCR and not culture or CrAg testing. The investigators reviewed the records of the 4 patients who had positive results by the Film Array ME Panel but negative results by the comparator PCR and determined that 2 were falsely negative by the comparator, and thus true positives detected by the Film Array ME Panel [6].
False-negative results of the Film Array ME panel owing to low burden of the disease have been demonstrated in a smaller preclinical study [7]. When the 16-target assay was performed on 342 stored CSF specimens, 8 of 14 (57%) CrAg positive specimens were positive by the Film Array ME panel, with 1 specimen that was CrAg negative/Film Array positive also testing positive by sequencing. The false-negative results came from specimens with relatively low CrAg titers and/ or high PCR crossing thresholds, and therefore were likely related to low burden of disease. This may also explain why the Film Array ME Panel performed well in subjects with first presentations of cryptococcal meningitis in a study performed in sub-Saharan Africa where the median quantitative culture was 8950 CFU/mL (IQR, 118-113 500 CFU/mL). Decreased sensitivity was observed in this study on follow-up CSF specimens obtained from therapeutic lumbar puncture in patients receiving appropriate antifungal therapy and presumably having lower fungal burden. In those with positive cryptococcal cultures and quantitative colony count <100 CFU/mL, the sensitivity was 50% (6/12) [8,9].
Another retrospective review identi?ed ?ve patients with cryptococcal meningoencephalitis, 4 of whom had a negative ME panel for Cryptococcus. All ?ve cases had positive serum CrAg, and three of ?ve had a positive CSF culture for Cryptococcus [10].
While the fungal cultures were positive on day 5 and day 7 respectively, time to culture positivity is dependent not only on fungal load but also on the volume of CSF inoculated, and hence, may not always represent burden of disease. On the other hand, the cryptococcal antigen LFA, which is performed with a fixed CSF volume, is more reproducible in quantifying fungal burden. The cryptococcal antigen LFA titre was positive in the undiluted sample for the both the patients.
Although the Biofire ME panel may potentially yield a rapid diagnosis, clinicians should be cautious and aware that a negative cryptococcal result on this molecular platform does not rule out cryptococcal meningitis. Therefore, in clinical scenario where there is a suspicion for cryptococcal meninigo encephalitis, assessment with multiple tests including CSF CrAg and culture should be performed to avoid delay in diagnosis.
Acknowledgement
The authors are thankful to Dr Naresh Trehan for his support.
References
- Rajasingham R, Smith RM, Park BJ, Nelesh P Govender, Tom M Chiller, David W Denning, et al. Global burden of disease of HIV associated cryptococcal meningitis: an updated analysis. Lancet Infect Dis. 2017; 17: 873-881.
- Brizendine KD, Baddley JW, Pappas PG. Predictors of mortality and differences in clinical features among patients with Cryptococcosis according to immune status. PLoS One. 2013; 8: e60431.
- Aye C, Henderson A, Yu H, Norton R. Cryptococcosis- the impact of delay to diagnosis. Clin Microbiol Infect. 2016; 22: 632-635.
- Nalintya E, Kiggundu R, Meya D. Evolution of cryptococcal antigen testing: what is new? Curr Fungal Infect Rep. 2016; 10: 62-67.
- Wootton SH, Aguilera E, Salazar L, Andrew C Hemmert, and Rodrigo Hasbun. Enhancing pathogen identification in patients with meningitis and a negative Gram stain using the BioFire Film Array® Meningitis/Encephalitis panel. Ann Clin Microbiol Antimicrob. 2016; 15: 26.
- Leber AL, Everhart K, Balada-Llasat JM, Jillian Cullison, Judy Daly, Sarah Holt, et al. Multicenter evaluation of BioFire Film Array Meningitis/Encephalitis panel for detection of bacteria, viruses, and yeast in cerebrospinal fluid specimens. J Clin Microbiol. 2016; 54: 2251-2261.
- Hanson KE, Slechta ES, Killpack JA, Jillian Cullison, Judy Daly, Sarah Holt, et al. Preclinical assessment of a fully automated multiplex PCR panel for detection of central nervous system pathogens. J Clin Microbiol. 2016; 54: 785-787.
- Rhein J, Bahr NC, Hemmert AC, Joann L Cloud, Satya Bellamkonda, Cody Oswald, et al. ASTRO-CM Team. Diagnostic performance of a multiplex PCR assay for meningitis in an HIV-infected population in Uganda. Diagn Microbiol Infect Dis. 2016; 84: 268-273.
- O'Halloran JA, Franklin A, Lainhart W, Burnham CA, Powderly W, Dubberke E. Pitfalls Associated With the Use of Molecular Diagnostic Panels in the Diagnosis of Cryptococcal Meningitis. Open Forum Infect Dis. 2017; 4: 1-3.
- Lewis PO, Lanier CG, Patel PD, Krolikowski WD, Krolikowski MA. False negative diagnostic errors with polymerase chain reaction for the detection of cryptococcal meningoencephalitis. Med Mycol. 2020; 58: 408-410.