
Case Report
Austin J Clin Case Rep. 2020; 7(2): 1169.
Phenytoin-Induced Purple Glow Syndrome
Shrilekha Rajesh Kombuwar, Venkateshwarlu Eggadi*, Bandaru Sharavana Bhava and Shravani Komuravelly
Department of Clinical Pharmacy & Pharm D, Vaagdevi College of Pharmacy, India
*Corresponding author: Venkateshwarlu Eggadi, HOD, Department of Clinical Pharmacy & Pharm D, Vaagdevi College of Pharmacy, Kakatiya University, Mahatma Gandhi Memorial Hospital, India
Received: July 28, 2020; Accepted: August 24, 2020; Published: August 31, 2020
Abstract
Background: Purple Glove Syndrome (PGS) is a rare complication of Intravenous (IV) phenytoin administration that is typified by delayed soft tissue injury of the skin adjacent to the site of IV phenytoin infusion. The clinical manifestation of PGS includes pain, edema, and purple-blue discoloration of skin tissue adjacent to the site of IV phenytoin infusion.
Case Report: A 37-year-old presented with swollen erythematous blisters following IV phenytoin administration for seizure episode.
Conclusion: In order to avert forthcoming complications, medical records should be upholded to decrease the probability of occurrence.
Keywords: Purple Glove Syndrome; IV Phenytoin; Purple Blue Discoloration
Introduction
Purple Glove Syndrome (PGS), a rare complication of Intravenous (IV) phenytoin administration that is characterized by [1,2,3] pain, edema, and purple-blue discoloration of skin tissue abutting to the site of IV phenytoin infusion which can occur in 6% of all phenytoin administrations. Clinically, PGS development encompasses 3 stages [4]. During the first stage, which appears within 2 to 12 hours following infusion of IV phenytoin, a dark purple-bluish discoloration of the skin develops around the site of IV phenytoin infusion [2]. In the second stage, which appears in the later 12 to 16 hours, edema develops and there is advancement of the dark purple-bluish discoloration around the skin enclosing the infusion site. During the lag stages of PGS, curing occurs with resolution of edema and receding of skin tissue discoloration. PGS has been outlined as being painful through all of its stages [1,4].
Treatment is supportive, and most cases resolve within days to weeks [5]. The intent was to present a case of a man who developed symptoms of purple glove syndrome following acute intravenous phenytoin administration.
Case Report
A 37-year-old male patient presented to the Mahatma Gandhi Memorial Hospital with complaints of seizure episode and loss of consciousness. He is a known case of alcohol liver disease (not on medication) and epilepsy (on medication) in the last 3 years but stopped 10 days back. He has a history of discoloration of eyes. He had been admitted at the primary care facility with complaints of frequent generalized tonic-clonic seizures for 5days. A provisional diagnosis of alcohol liver disease with seizures with alcohol withdrawal in view of the history and presence of complaints. The patient was administered intravenous phenytoin (100mg/TID) following T. Ursodioxycolic acid (300mg/BD), T. Pantoprazole (40mg/OD), Inj. Ceftriaxone (1g/BD), T. Librium(25mg/OD), T. Benfothiamine (100mg/OD). A day following IV phenytoin administration, patient developed dark blue and purple in color, swollen, erythematous, blisters, blebs ruptured and wall necrotic patch were observed on his left wrist up to elbow on his left hand (Figure 1).
Outcome and Follow-up
After IV phenytoin induced dark blue and purple in color, swollen, erythematous, blisters, blebs ruptured and wall necrotic patch on the left limb was observed the frequency of IV phenytoin was reduced to twice daily. For treatment the patient was shifted to general surgery ward and the wound care team used a combination of dry dressing material and left forearm elevation active finger movement to reduce edema and to avoid further secondary infection. Patient cannula was shifted to right limb for further treatment. The patient’s injury was healing (subsiding swelling and receding of the area of discoloration) by the time he was discharged.
Discussion
Purple Glove Syndrome (PGS), a severe adverse reaction which is not well assumed. The phenomenal indications include skin discoloration, pain, abscess formation, sloughing, ulceration. Very unlikely PGS may progress to necrosis, ischemia, vascular compression, compartment syndrome which may necessitate surgical interventions such as fasciotomy, skin grafting, or rarely amputation [1,2]. PGS has been found to perpetuate a patient’s hospital stay [3]. Risk factors for PGS after IV phenytoin administration include old age, female sex, peripheral vascular disease, sepsis, history of chronic debilitating disease, number of doses, high dose (>15-20 mg/kg body weight), higher infusion rate. The pathogenesis of PGS is not well assumed but histological examination has implied thrombosis might play a role in pathophysiology. Initial management includes analgesics, elevation of impaired limb, compression, kneading and gentle heat. Management is essentially supportive. There are no archives where of PGS reported in case of oral medication heretofore but possibility of occurrence is existent [5].
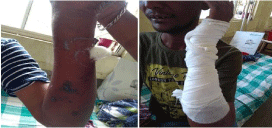
Figure 1: Left hand depicts multiple blisters with purple-blue discoloration,
erythematous skin, blebs ruptured and wall necrotic patch are observed.
Conclusion
PGS may be obviated by employing the use of a substitutive agent which is analogous in terms of efficacy. Early identification and prompt monitoring & management are fundamental for restoration of event occurred. Although phenytoin has a significantly associated with risk of PGS. Increased awareness regarding risk factors of induction and to curtail the morbidity of this pernicious adverse drug reaction.
References
- Hanna DR. Purple glove syndrome: A complication of intravenous phenytoin. J Neurosci Nurs. 1992; 24: 340-345.
- Chokshi R, Openshaw J, Mehta NN, Mohler E. Purple glove syndrome following intravenous phenytoin administration. Vasc Med. 2007; 12: 29-31.
- O’Brien TJ, Cascino GD, So EL, Hanna DR. Incidence and clinical consequence of the purple glove syndrome in patients receiving intravenous phenytoin. Neurology. 1998; 51: 1034-1039.
- Burneo JG, Anandan JV, Barkley GL. A prospective study of the incidence of the purple glove syndrome. Epilepsia. 2001; 42: 1156-1159.
- Garbovsky LA, Druheller BC, Perrone, J. Purple glove syndrome after phenytoin or fosphenytoin administration: review of reported cases and recommendations for prevention. J Med Toxicol. 2015; 11: 445-459.