
Case Report
Austin J Clin Case Rep. 2020; 7(2): 1170.
A 61-Year-Old Man with a Concomitant Chronic Lymphocytic Leukemia and Renal Cell Carcinoma
Lujain Hamdan*, Kinda Mohammad and Firas Hussein
Department of Clinical Hematology, Tishreen University Hospital, Lattakia, Syria
*Corresponding author: Lujain Hamdan, Department of Clinical Hematology, Tishreen University Hospital, Lattakia, Syria
Received: July 28, 2020; Accepted: August 25, 2020; Published: September 01, 2020
Abstract
The association between Renal Cell Carcinoma (RCC) and Non-Hodgkin's Lymphomas (NHL) has been reported in the past years more than expected. In this case, a 61-year-old- patient was diagnosed with Chronic Lymphocytic Leukemia (CLL) and during his work up, RCC was found out at the same time. Previous studies have attributed this association to specific genetic mutations, previous chemotherapy/radiotherapy, hormonal-environmental factors or immunomodulation. However, the accurate mechanism of this connection remains unclear. Through this case we aim to draw the attention of clinicians for further investigations on this unexplained connection.
Keywords: Non-Hodgkin's Lymphoma; Renal Cell Carcinoma; Simultaneous Association
Abbreviations
NHL: Non-Hodgkin’s Lymphoma; RCC: Renal Cell Carcinoma; CLL: Chronic Lymphocytic Leukemia; HM: Hematologic Malignancies
Introduction
Chronic Lymphocytic Leukemia is a monoclonal disorder characterized by a progressive accumulation of functionally incompetent lymphocytes. CLL is the most common form of leukemia found in adults in Western countries. The overall survival rate ranges between 5 to 10 years, although some patients have a lower life expectancy [1].
Renal Cell Carcinoma (RCC) is a result of malignant proliferation of the epithelial cells of proximal convoluted tubule of the nephron, and it accounts for 95% of the malignant neoplasm of kidney [2].
The authors described a possible association of RCC and lymphoid malignancies. The co-existence of both malignancies in the same individual has been reported more than expected in the general population over the past 20 years [3,4]. Several epidemiological studies have revealed that the observed-to-expected ratio for occurrence of RCC in NHL patients was 1,86-fold greater than the expected rates in the general population, while the risk of NHL in the RCC patients was 2.67 times higher [5,6]. This article discusses a case of simultaneous occurrence of CLL and RCC in the same patient.
Case Report
A 61-year-old, light alcoholic, non-smoker male with no past medical history, except for his mother who had breast cancer, visited the hematology clinic with right lower quadrant pain for the past month, accompanied by anorexia, polyuria and dry cough. He also mentioned mild weight loss in the past two months. At administration, his blood pressure was 120/70 mm Hg, his body temperature was 37°, his heart rate was 81 bpm and his oxygen saturation was 98%.
His blood count parameters were as follows: Hemoglobin (Hb): 10.3 g/dl, MCV: 73 FL, Leukocyte: 21×103/mm3, Lymphocyte: 15.5×103/mm3, Platelet Count (PLT): 210×103/mm3. Erythrocyte Sedimentation Rate (ESR) was 103 mm/h, C - reactive protein (CRP) was 68 mg/L (normal range: 0-5).
Creatinine and urea values were 1.5 mg/dl and 36 mg/dl respectively, uric acid was 7.2 mg/dl and there were 15-20 red blood cells in urine sample. All other laboratory tests including serum electrolytes, liver function tests and coagulation profile were normal. Direct and indirect antiglobulins tests were negative, reticulocyte correction index was 0.08 and detailed laboratory investigations including protein electrophoresis and tumour markers were unremarkable.
On peripheral blood smear there was an increase in white blood cells with a majority of matured lymphocytes, red blood cells were hypochromic with variation in size and shape, and platelets were normal.
Immunophenotyping demonstrated the presence of CD5, CD19, CD20, CD22, CD23, CD45, HLA-DR, and Kappa light chain expression, confirming the diagnosis of B-cell chronic lymphocytic leukemia. Then, a complete computed tomography CT was made to determine the clinical stage. It showed several axillary and mediastinal lymphatic nodes measuring between 1 and 2.2 cm. The liver was normal with a few small low-density foci, which were likely to be biliary cysts. The spleen was enlarged (around 17 cm) with a small splenunculus. And finally, a large right infrarenal mass, measuring 7*6*7 cm with an increased uptake, invades the right psoas muscle (Figure 1, Figure 2).
The previous data classified the patient as a stage (A) on Binet classification. Accordingly, he was put on ‘watch and wait’ strategy.
The patient then was elected for surgery to investigate the kidney mass. The mass was removed alongside the right kidney. The kidney was dissected from neighboring organs and the ureter was ligated (Figure 3).
Histopathology of the resected mass was consistent with RCC (Clear cell Renal Cell Carcinoma). It measured 8 cm with an infiltration to the fibrous renal capsule and the perirenal fat but not beyond Gerota's fascia. The mass also invaded one small renal vein branch in the renal hilus. The surgical margins were free and no lymph nodes were detected in the specimen.
A PET/CT MULTI SLICE (positron emission tomography- computed tomography) was performed later to determine the tumour staging accurately. It showed no metastases, with no abnormal uptake.
Accordingly, the patient was a stage 3 RCC (T3a, N0, M0). Thus, there was no need for immunotherapy or target therapy.
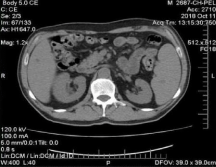
Figure 1: Preoperative CT scan, coronal, showing a right infrarenal mass.
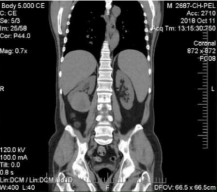
Figure 2: Preoperative CT scan, axial, shows a right infrarenal mass.
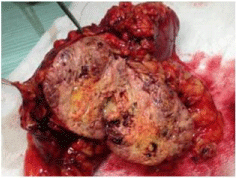
Figure 3: The resected infrarenal mass.
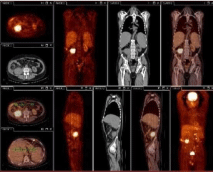
Figure 4: PET/SCAN after one year of nephrectomy, shows local relapse in the right kidney area.
The patient was monitored with blood tests and imaging studies every 3 months. Then after a year with no complications, a second PET/CT was performed (Figure 4). It showed local relapse: a 6 cm mass in the right renal area, with an uptake up to (10 SUV), invading the right psoas muscle.
Based on the new findings, another operation was performed to eradicate the relapsed mass, and the patient was put on Pazopanib (a terosine kinase inhibitor). Currently, he's being followed up by our department.
Discussion
The association between RCC and NHL has been studied over the past two decades in many papers. The repetitive incidence of these two diseases presumes the existence of a definite relation between them [3,7].
Rabbiani et al. identified the five most common second malignancies in 27% of patients with RCC, and those are prostate, breast, colon, bladder and NHL [8]. The co- existence of RCC with NHL is more common than Hodgkin lymphomas. However, the occurrence of RCC with CLL is rare [4,7].
Literature data showed that RCC and HM incidence may be synchronous or asynchronous [7,9]. According to kunthur and Dutcher et al. reviews through literature, it was found that the most common pattern of diagnosis was a HM followed by RCC, secondly concurrently and the least observed sequence was RCC first [6,10].
The authors reported that these patients have common clinical characteristics. It was found that the large majority of these HMs are of B-cell origin, with a higher risk of extranodal lymphoma [3,5,10]. Moreover, the observations showed male predominance in patients with both diseases (2,2:1) comparing to the expected male ratio of each disease [3,5,10].
Despite all the continuous studies, there is no definite etiology that could explain the relation between RCC and HMs. However, some possible mechanisms have been suggested to clarify this link, including prior treatment modalities, advanced imaging studies, immunosuppression due to underlying lymphoproliferative disorder and environmental factors (such as geography and probable exposure to carcinogen) [2,6,11]. Unfortunately, these mechanisms might only explain the reason behind asynchronous RCC and NHL, but data showed, similarly to the patient in this case report, that sometimes the incidence of these disorders might happen simultaneously concomitant. Thus, it can't be attributed to the previous etiologies [3].
Further investigations were made and different hypothesis were suggested. Yagisava et al. reported a patient with lymphoma and RCC in whom there were multiple immunological disorders [12]. Sakai et al. suggested that IL-6 and related cytokines synthesis by RCC cells could enhance malignant lymphoroliferation [13].
Other than that, Several studies have suggested a viral etiology, such as Epstein-Barr Virus (EBV) and Human T- Lymphotropic Virus 1 (HTLV1) in some lymphomas, mouse mammary tumor virus in NHL, and Human papillomavirus (HPV) in cervical, laryngeal and nonmelanomic skin cancer, while the role of HPV in RCC is still contreversial [3,6].
A review of the literature reveals some common genetic mutations in RCC and NHL, include deletions of 3P (seen in 95% of the sporadic clear renal cell carcinoma), abnormalities in 11p, chromosome 13 and deletions in 17p. Deletions and mutations of the VHL locus (a tumor suppressor gene) on 3p are demonstrated in Von Hippel- Lindau syndrome, in which affected individuals develop RCC at a rate of 40%. Whether identified genes are involved is yet to be determined. Cytogenetic analysis of NHL by ratio painting and comparative genomic hybridization has revealed unsuspected chromosomal abnormalities including 17p deletions, trisomy 7 and mutations in 3p and p53. [3,5,6].
Another review was made by Dr. Peter H. Wiernika, he noticed that there is a previously unrecognized genetic connection between RCC and HMs, primarily lymphoid malignancies. Clinicians have previously shown a similar relationship between breast cancer and lymphoid malignancies [10]. It's noticeable that our patient's mother had a history of breast cancer, and breast and renal cancer are both adenocarcinomas. Molecular genetic analysis is finding new pathways that may connect RCC and HM. For example, mutation in the phosphatase and tensin homolog (PTEN) pathway has been implicated in familiar cancer syndromes, deletion of PTEN may have a role in lymphoid leukemias and lymphoma, and loss of PTEN can lead to activation of mammalian Target of Rapamycin (mTOR), a new therapeutic target for RCC [10]. The exact mechanism remains unclear, and further investigations should be done.
Conclusion
Clinicians should be alerted to the possibility of co- existence of RCC and NHL even though it’s a rare event, and further studies are required to elucidate a common etiology for these disorders.
Acknowledgement
We would like to thank Dr. Firas Hussein for his guidance. We also would like to thank our parents for providing help all the way.
References
- Nabhan C, Rosen ST. Chronic lymphocytic leukemia: a clinical review. JAMA. 2014; 312: 2265-2276.
- Ghalamkari, Marziye & Mirzania, Mehrzad & Khatuni, Mahdi. A patient with Multiple myeloma and Renal cell carcinoma. International journal of hematology-oncology and stem cell research. 2016; 10: 56-60.
- Serefhanoglu S, Buyukasik Y, Goker H, Safak Cavus Akin, Serkan Akin, Nilgun Sayinalp, et al. Concomitant renal cell carcinoma and lymphoid malignancies: a case series of five patients and review of the literature. Med Oncol. 2010; 27: 55-58.
- Zabrocka, Ewa & Sierko, Ewa & Wojtukiewicz, Marek. A case of renal cell carcinoma metastases to the neck after long-term latency in the setting of chronic lymphocytic leukemia progression. European Journal of Oncology. 2016; 21: 251-253.
- Uz B, Dolasik I, Ozlem Ucer, Adile Ferda Dagli, and Sercan Simsek. Chronic lymphocytic lymphoma and concomitant renal cell carcinoma (Clear Cell Type): review of the literature. Leuk Res Rep. 2016; 6: 8-10.
- Kunthur A, Wiernik PH, Dutcher JP. Renal parenchymal tumors and lymphoma in the same patient: case series and review of the literature. Am J Hematol. 2006; 81: 271-280.
- Sargin G, Yavasoglu I. Renal cell carcinoma and chronic lymphocytic leukemia. Med. 2013; 30: 547-548.
- Rabbani F, Reuter VE, Katz J, Russo P. Second primary malignancies associated with renal cell carcinoma: influence of histologic type. Urology. 2000; 56: 399-403.
- Dutcher, Janice & Wiernik, Peter H & Varella, Leticia & Chintapatla, Rangaswamy. Occurrence of renal cell carcinoma and hematologic malignancies (predominantly lymphoid) in individuals and in families. Familial cancer. 2016; 15: 677-687.
- JP Dutcher, PH Wiernik. Renal cell carcinoma in patients with a personal or family history of hematologic malignancies.Clin. Adv. Hematol. Oncol. 2015; 13: 392-397.
- Deeb R, Zhang Z, Ghanem T. Metastatic renal cell carcinoma to the parotid gland in the setting of chronic lymphocytic Leukemia. Case Rep Med. 2012.
- Yagisava K, Ohno Y, Toba K, et al. There cases of malignant lymphoma accompanied by renal cell carcinoma. Rinsho Kets-ueki. 2001; 42: 616-620.
- Sakai A, Kawano M, Kuramoto A. Interleukin-6 produced by renal cell carcinoma cells and progression of multiple myeloma. New Engl J Med. 1991; 324:1893-1894.