
Case Report
Austin J Clin Case Rep. 2020; 7(4): 1177.
Elevated Ferritin in ESRD Patients from Developing Countries
Niharika Singh¹*, Max Rosenthaler¹ and Charles Milrod²
¹Max Rosenthaler is a Fourth-year Medical Student at Boston University School of Medicine in Boston, Massachusetts, USA
²Charles Milrod is a PGY-2 Internal Medicine Resident at Boston Medical Center, USA
*Corresponding author: Niharika Singh, Boston University School of Medicine in Boston, USA
Received: October 19, 2020; Accepted: October 30, 2020; Published: November 06, 2020
Abstract
A patient with End Stage Renal Disease (ESRD) on dialysis presents with heart failure with reduced ejection fraction in the setting of an upper respiratory infection and is found incidentally to have severely elevated ferritin (10,284 mcg/L). Iron studies revealed a pattern consistent with iron overload (iron of 111, transferrin saturation of 64%). On taking a thorough history, the patient is found to have recently had an ICU admission in his home country of the Dominican Republic (DR) with unclear admitting diagnosis and treatment. In the context of his recent hospitalization in the DR, the patient is determined to likely have iatrogenic iron overload from iron supplementation or blood transfusions during his ICU stay. There was concern for infiltrative cardiomyopathy secondary to hemochromatosis; however, he was found to have a new diagnosis of amyloidosis. The patient’s ferritin decreased to 1,709 without chelation therapy and was discharged after stabilization of his heart function. This case report illustrates the importance of elucidating a patient’s access to renal replacement therapy while abroad.
Keywords: Iatrogenic; Hemochromatosis; Infiltrative Cardiomyopathy; Global Health
Introduction
Anemia is a common complication of chronic kidney disease. Current standard of care is to administer Erythropoiesis-Stimulating Agents (ESAs) to most CKD patients who have a hemoglobin (Hb)<10 g/dL. An exception is made for patients with active malignancy or a recent history of malignancy, or who have had a stroke because such patients may be at higher risk for adverse effects from ESAs. Silverberg et al. showed that the combination of low-dose EPO and intravenous iron has an additive effect on the correction of anemia in pre-dialysis patients with chronic renal failure compared to intravenous iron alone [1]. The target hemoglobin goal in dialysis patients is >10 g/dl.
Lack of access to Renal Replacement Therapy (RRT) is a global problem, with projections that only one quarter to one half of individuals who need renal replacement therapy receive it [2]. Lower-income countries face some fundamental challenges: reducing infectious diseases that can lead to acute kidney injury; establishing the infrastructure and cultural acceptance necessary to perform both deceased donor and living-related donor kidney transplants; and attempting to deliver lower-cost and less water-intensive therapies, such as peritoneal dialysis and home hemodialysis [2].
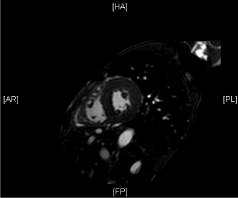
Figure 1: Cardiac MRI End Diastolic Short Axis.
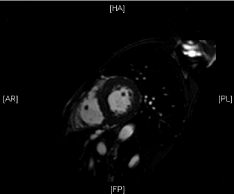
Figure 2: Cardiac MRI End Systolic Short Axis.
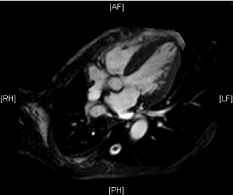
Figure 3: Cardiac MRI End Diastolic Long Axis.
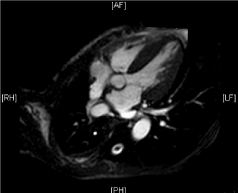
Figure 4: End Systolic Long Axis.
Society Guideline
Normal Values
ACP
Transferrin Saturation < 55%
Ferritin < 300 mcg/L (males), Ferritin < 200 mcg/L (females) [5]
AASLD
Transferrin Saturation < 45%
Ferritin < 200 mcg/L (males), Ferritin < 150 mcg/L (females) [6]
Table 1: The Society Guidelines and Iron values mentioned in the below table.
However, even in those patients receiving RRT, lack of access to ESAs continues to be a problem in developing countries. Despite the paucity of research into ESRD management in resource-limited areas, Maïz et al. found that in Tunisia, only 10.8% of patients with ESRD related anemia on RRT were receiving EPO, whereas 38% of anemic patients received blood transfusions and 42% of anemic patients received intravenous iron alone [3]. Even those that received EPO were receiving low doses (< 4,000 units/week) that did not maximize the degree to which hemoglobin counts increased [3]. RRT centers cited the prohibitive cost and limited availability of EPO as the primary reason that routine supplementation was not more commonplace [3].
Intravenous iron or blood transfusions are not without side effects. The human body can typically only clear 1 mg of iron per day. Excess iron is stored in the body as ferritin. Eventually, a sufficient iron load can produce symptomatic iron overload, which can manifest with liver dysfunction, cardiac toxicity, endocrine dysfunction (diabetes, hypogonadism), skin pigmentation, and arthropathy [4].
Case Report
Mr. P is a 63-year-old Spanish-speaking man from the Dominican Republic presenting with 3 episodes of acute non-bloody, non-bilious emesis and dyspnea at rest for 1 day. He also notes a nonproductive cough and weakness for the past 3 weeks. His past medical history is notable for end stage renal disease secondary to multiple myeloma/ cast nephropathy on hemodialysis, multiple myeloma (IgG Kappa), aortic regurgitation secondary to rheumatic valvulopathy (LVEF 55% 3/2019), and prior NSTEMI. The patient recently returned from his mother’s funeral in Santo Domingo, Dominican Republic. While in the DR, he developed a cough and weakness and was admitted to the ICU for 7 days and was discharged 15 days prior to admission. Patient does not know what was wrong with him or what procedures were done. He was feeling better until 4am on the day of admission, when he awoke with episodes of emesis with subsequent dyspnea that felt like he was “gasping for air”. He denies fevers, chills, sore throat, chest pain, abdominal pain, and diarrhea.
In the ED, he was afebrile, hypertensive to 170/90s, tachycardic to 100s, and saturating 95-96% on 2.5 L O2. His labs were notable for a hemoglobin of 9.3 with a baseline of 11, potassium of 5.3, BUN of 43, Cr of 7.75, and elevated troponins at 0.783 that down trended to 0.746. He was found to be rhino/enterovirus positive. A chest X-ray was performed, which showed a large right-sided effusion, which appeared stable from previous chest X-rays. A CTPA was performed which showed no pulmonary embolism and loculated fluid at the right lung base. On the floor, his heart rate improved to the 60s.
On day 2 of his admission, the pulmonary team was consulted for chest tube placement for the loculated pleural fluid. The pleural fluid was found to be exudative based on Light’s criteria of LDH>2/3 upper limit of normal, with lymphocyte predominance (85%). The most likely etiology was uremic pleurisy, although TB and lymphoma could not be ruled out.
On day 3 of his admission, iron studies were ordered, which showed an iron level of 111, ferritin of 10,284 mcg/L, TIBC of 174, and transferrin saturation of 64%. Over the last 5 years, this patienthad elevated ferritin varying between 600 – 1200; however the transferrin saturation had always been normal before this hospitalization. He received an echocardiogram showing a newly reduced Ejection Fraction (EF) of 30%, down from 55% from 10 months ago. A cardiology consult was concerned for an infiltrative process due to the rapid decrease in EF, specifically hemochromatosis, due to the high ferritin level, as well as amyloid and light-chain deposition.
A cardiac MRI was performed without contrast due to renal disease and showed no iron overload. Amyloidosis could not be ruled out due to the lack of contrast. A fat pad aspirate was performed, which resulted positive red stain consistent with amyloid.
On day 6 of admission, Mr. P’s ferritin decreased to 1,709 from 10,284 without intervention, most consistent with iatrogenic iron overload from dialysis at Santo Domingo.
Twelve days after the day of admission, the patient was discharged. For his newly reduced EF secondary to amyloidosis, outpatient follow up with cardiology and outpatient hematology/oncology were scheduled. His shortness of breath was likely secondary to rhino/enterovirus, and pulmonology follow up was scheduled for monitoring his right pleural effusion.
Differential Diagnosis
When to worry about Iron Overload
Society recommendations vary about when iron studies are concerning for iron overload, with the American Association for the Study of Liver Diseases (AASLD) having the most sensitive threshold and the American College of Physicians (ACP) having a more specific threshold.
Differential Diagnosis for Elevated Ferritin
Chronic inflammation: Chronic inflammatory conditions are the most common cause of a mildly elevated ferritin. Ferritin is an acute phase reactant, and thus rises in inflammatory states regardless of iron status. Typically, ferritin will be less than 2-3 times normal. Therefore, chronic inflammation is unlikely to fully account for the level of elevation in our patient, though it could be a component.
One inflammatory condition to consider in our patient with a history of recent ICU admission with possible transfusion of blood products in a developing country is HIV infection. However, ferritin levels in HIV infection are usually < 500 mcg/L and unlikely the cause of such a drastic elevation. In this patient, HIV serologies were negative.
Malignancy
Malignancy is associated with increased ferritin, which is attributed to being in a chronic inflammatory state. Malignancy is a unique inflammatory state, however, in that it occasionally can result in severe elevations into the several thousands, but more commonly causes mild elevations [7]. In one cohort study analyzing the causes of ferritin elevations > 1,000 mcg/L in hospitalized patients, malignancy was the most common cause (153/627) [7]. In our patient with a history of multiple myeloma, malignancy should definitely be considered a possible cause of severely elevated ferritin. However, typically in malignancy transferrin saturation is normal or low, and a ferritin level > 10,000 mcg/L would be rare.
Hemophagocytic lymphohistiocytosis
Hemophagocytic Lymphohistiocytosis (HLH) is an inflammatory condition which usually presents with a ferritin level of 5000-20,000 ng/ml. It occurs with acute severe illness with fever, hepatosplenomegaly, rash, neurologic findings, and pancytopenia. It is more common in children, but can happen in any severely ill person. This syndrome characteristically has visible phagocytosis of red blood cells on peripheral smear, although this is not a required finding. Although this patient had a ferritin level of 10,284 ng/ml, he lacked fever, other clinical signs of HLH, and overall was not acutely ill enough to suspect HLH.
Iron Overload Syndromes
Iron overload syndromes in the aforementioned cohort study were the second most common cause of ferritin elevations > 1,000 (136/627) [7].
Hereditary hemochromatosis is characterized by an elevated ferritin and transferrin saturation. In population based-studies, the majority of patients with iron overload secondary to hereditary hemochromatosis will have ferritin levels < 1,000, but can have wide variation presenting values [8]. Patients present with many different constellations of clinical symptoms, but it is most commonly associated with chronic liver disease, infiltrative cardiomyopathy, joint disease, type 2 diabetes, and skin hyperpigmentation [3]. Although consistent with our patient’s elevated transferrin saturation, a new presentation as a 63 year old male without any other new clinical signs would be unusual.
Iatrogenic iron overload from pRBC transfusion or iron supplementation can also present with a wide range of ferritin levels from slightly elevated to a ferritin in the tens of thousands. It is usually associated with an elevated transferrin saturation, but not always. Typically this is evident from a history of blood and iron transfusions. This patient is at high risk given that he has ESRD on HD and has intermittently been receiving care in low resource areas in the Dominican Republic. Given his recent ICU admission in a foreign country, level of elevation > 10,000, and increased transferrin saturation this is the most likely diagnosis.
Discussion
Lack of access to RRT is a global problem, and even in those patients receiving RRT, lack of access to ESAs continues to pose a problem. Low resource areas without access to erythropoietin analogues due to prohibitive cost will often opt to transfuse pRBCs for symptomatic anemia or heavily supplement IV iron with the goal of stimulating erythropoiesis. Excess iron can manifest as symptomatic hemochromatosis, including liver dysfunction, cardiac toxicity, endocrine dysfunction (diabetes, hypogonadism), skin pigmentation, and arthropathy [4]. Although this patient did not have hemochromatosis causing an infiltrative cardiomyopathy, it was a diagnosis that had to be ruled out. As evidenced by this patient, it is always important to consider how patients access RRT when outside of the US and their risk of iatrogenic iron overload.
References
- Silverberg DS, Blum M, Agbaria Z, V Deutsch, M Irony, D Schwartz, R Baruch, et al. The effect of i.v. iron alone or in combination with low-dose erythropoietin in the rapid correction of anemia of chronic renal failure in the predialysis period. Clin Nephrol. 2001; 55: 212-219.
- Wetmore JB, Collins AJ. Global challenges posed by the growth of end-stage renal disease. Ren Replace Ther. 2016; 2: 15.
- BEN Maiz H, Abderrahim E, Zouaghi K. Anemia and End-Stage Renal Disease in the Developing World. Artif Organs. 2002; 26: 760-764.
- Siddique A, Kowdley K V. Review article: the iron overloads syndromes. Aliment Pharmacol Ther. 2012; 35: 876-893.
- Qaseem A, Aronson M, Fitterman N, Snow V, Weiss KB, Owens DK. Screening for hereditary hemochromatosis: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2005; 143: 517-521.
- Bacon BR, Adams PC, Kowdley K V, Powell LW, Tavill AS. Diagnosis and management of hemochromatosis: 2011 practice guideline by the American Association for the Study of Liver Diseases. Hepatology. 2011; 54: 328-343.
- Moore CJ, Ormseth M, Fuchs H. Causes and Significance of Markedly Elevated Serum Ferritin Levels in an Academic Medical Center. JCR J Clin Rheumatol. 2013; 19: 324-328.
- Olynyk JK, Cullen DJ, Aquilia S, Rossi E, Summerville L, Powell LW. A Population-Based Study of the Clinical Expression of the Hemochromatosis Gene. N Engl J Med. 1999; 341: 718-724.