
Research Article
Austin J Clin Case Rep. 2021; 8(7): 1222.
The Current Role of Immunotherapy in mCRPC: A Systematic Review
Dalibey H1,2,3, Hansen TF1,2 and Zedan AH1,2*
¹Department of Oncology, Vejle Hospital, Vejle, Denmark
²Institute of Regional Health Research, University of Southern Denmark, Denmark
³University of Southern Denmark, Odense, Denmark
*Corresponding author: Zedan AH, Department of Oncology, Vejle Hospital, Beriderbakken 4, 7100 Vejle, Denmark
Received: May 31, 2021; Accepted: June 29, 2021; Published: July 06, 2021
Abstract
Background: The development of immunotherapy has shown promising results in several malignant diseases, including prostate cancer, calling for a systematic review of the current literature. This review aims to evaluate the present data and prospects of immune checkpoint inhibitors in metastatic Castration Resistant Prostate Cancer (mCRPC).
Methods: Articles were identified via a systematic search of the electronic database Pubmed, in accordance with the PICO process and following the PRISMA guidelines. Articles in English studying immune checkpoint inhibitors in patients with mCRPC published between March 2010 and March 2020 were eligible for inclusion. Endpoints of interest were Overall Survival (OS), Progression-Free Survival (PFS), clinical Overall Response Rate (ORR), and Prostate-Specific Antigen (PSA) response rate.
Results: Ten articles were identified as eligible for inclusion. The studies primarily explored the use of Ipilimumab, a CTLA-4 inhibitor, and Pembrolizumab, a PD-1 inhibitor. These drugs were both used either as monotherapy or in combination with other treatment modalities. The largest trial included in the review demonstrated no significant difference in overall survival between the intervention and placebo. However, two studies presented promising data combing immunotherapy and immune vaccines. Grade 3 and 4 adverse events ranging from 10.1% to 82.3%, whit diarrhea, rash, and fatigue were the most frequently reported. Forty relevant ongoing trials were identified exploring immunotherapy with or without a parallel treatment modality.
Conclusion: Overall, the current data shows that the effect of immune checkpoint inhibitors as monotherapy may have limited impact on mCRPC, and the results from ongoing combinational trials are eagerly awaited.
Keywords: Cancer-immunotherapy; Immune-checkpoint; Prostaticneoplasms; Castration-resistant
Introduction
Prostate Cancer (PCa), the fourth most common cause of cancerrelated deaths in men worldwide, is a high mortality disease calling for improvements [1]. Treatment options for early and localized stages of PCa are promising and the disease generally develops slowly. Symptoms, however, are typically interpreted as age-related, resulting in PCa often being detected in its advanced stages. Treatment with Androgen-Deprivation Therapies (ADT) can initiate antitumor activity in the initial phase of PCa, however, the tumor cells eventually stop responding to ADT and progress to a state referred to as Castration-Resistant Prostate Cancer (CRPC).
Despite the many therapeutic choices introduced in the past two decades, CRPC is still considered terminal, with a median survival of only 16-21 months [2]. The poor prognosis may partly be explained by both the aggressiveness of the disease and the limitations of the available treatments.
Many alternative therapies have been investigated over the past years. However, one treatment modality in particular has dominated the past century; immunotherapy. In contrast to chemotherapy, which causes immunosuppression, immunotherapy cooperates with the immune system to fight cancer. Treatment with immunotherapy is able to target a wide variety of regulatory pathways, with individual drugs aiming for different targets.
In general, the immune system’s activity must be carefully regulated to ensure that activation only occurs when required. The immune system has several “off switches” responsible for its inactivation. Two of them; Cytotoxic T-Lymphocyte-Associated Protein 4 (CTLA-4) and programmed cell death protein 1 (PD- 1), are currently targeted by immunotherapy. These off switches, or pathways, Called Immune Checkpoints (ICP) have been well documented. ICP’s comprise a variety of regulatory pathways that are crucial for the initiation, duration, and regulation of the immune response. The immune system also uses the ICP’s to distinguish between normal and apoptosis-demanding cells.
In cancer diseases, tumor cells evade the immune system by overexpressing ICP receptors, resulting in the inhibition of T-cells. The understanding of these pathways has led to the discovery of a new treatment option; ICP Inhibitors (ICPIs). The ICPIs work by inhibiting the ICP receptors on cancer cells, thereby making tumor cells visible for the immune system [3]. The ICPIs have become a cornerstone in the treatment of cancers such as lung cancer and metastatic melanoma [4]. A phase-3 clinical trial in metastatic melanoma patients showed a significant improvement in survival with the drug Ipilimumab [5]. The subsequent FDA approval of this fully human CTLA-4 inhibiting monoclonal antibody paved the way for a number of clinical trials in many cancer types, including PCa.
Tumor cells in PCa exploit a broad range of mechanisms to evade activation of the immune system. A study has shown that activation and infiltration of T-cells and inflammatory cells in PCa tissue may mediate antitumor responses [6].
The first ever immunotherapy approved for the cancer treatment was the Sipuleucel-T injection, popularly known as “immune vaccine” [7], and for metastatic CRPC (mCRPC). Since its release in 2009, the therapy has been heavily elucidated through clinical research. However, the treatment has been poorly adopted due to it being effective only in a narrow group of patients and its high cost.
Recent promising results from immunotherapy have raised hopes for potential benefits from ICPIs in mCRPC. This has resulted in the initiation and publication of several mCRPC studies, yet with no recent review summarizing these results.
The aim of this review is to systematically identify and summarize the literature on the current status and future perspectives of ICPI treatment of mCRPC.
Checkpoint inhibitors
Research and understanding of the immune system are far from fully elucidated, and the current field of ICP-research is primarily focused on CTLA-4 and PD-1.
CTLA-4
CTLA-4 is an ICP receptor present on the cell membrane of activated T-cells. With CTLA-4’s ability to downregulate effector T-cells’ activity and increase the activity of regulatory T-cells, CTLA- 4 plays an important role in the modulation of immune response [8,9].
The activation of T-cells requires signaling from two independent origins. Firstly, the T-cells are introduced with an antigen from the Antigen Presenting Cell (APC). Secondly, the binding of costimulatory receptor CD28 on T-cells to CD80/CD86 on the APC, resulting in an increase in the proliferation of T-cells and differentiation of T-cells into T-memory cells [10,11]. CTLA-4, a CD80 homologue, then binds to CD80/CD86 with greater affinity than its competitor CD28. This leads to the inhibition of T-cells, and subsequently avoiding hyperactivation of the immune system. The binding of CD28:CD80/86 initiates a positive response in which CTLA-4 is upregulated on the cell membrane [12]. Cancer cells disturb this pathway to their advantage, resulting in an immunosuppressive environment that prevents antitumor activity [13].
By inhibiting the CLTA-4 pathway with immunotherapy, it may be possible to hinder the immune-inactivated environment created by the cancer cells. This treatment may furthermore lead to activation and proliferation of effector T-cells and thereby increasing antitumor activity [14,15].
PD-1/PD-L1
The PD-1/ Programmed Death Ligand 1 (PD-L1) pathway is another crucial pathway for the regulation of T-cell activity. Although similar to the CTLA-4 pathway, PD-L1 are expressed on numerous cells types and act in the peripheral tissue, whereas CTLA-4 are solely found on T-cells [16,17].
PD-1 is a transmembrane glycoprotein receptor expressed on CD4+ and CD8+ activated T-cells, B-lymphocytes, natural killer cells, and monocytes. The PD-1 receptor is a part of the CD28 superfamily and functions as an ICP and thereby decreases T-cell activity [18]. PD-1 has two primary ligands: programmed death-1 ligand 1(PDL1) and Programmed Death-Ligand 2 (PD-L2). When comparing the receptor affinity of PD-L1 and PD-L2, studies have shown that PD-L1 has a three times greater affinity to PD-1 than PD-L2 [19]. The PD-1/ PD-L1 checkpoint primarily functions as a regulator, preventing unwanted autoimmune-responses [20]. The expression of PD-L1 is initiated by interleukins that are produced when an interaction occurs between a T-cell and an antigen from an APC. The sudden expression of PD-L1 allows for the binding of PD-L1 to PD-1 and this binding initiates T-cell inhibition [21,22]. By overexpressing PD-L1, tumor cells hereby evade immune system activation and create an immunosuppressive microenvironment, favorable for their uncontrollable growth [23].
Inhibiting the PD-1/PD-L1 checkpoint with monoclonal antibodies may potentially reintroduce the T-cell antitumor activity and diminish the immunosuppressive microenvironment [24-26].
By creating a specific monoclonal antibody to inhibit the PD-1 immune checkpoint, the immune system’s awareness of cancer cells increases, and cytotoxic T-cells can once again react to the malignant tumor cells [27].
Method
This systematic review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [28,29]. The PRISMA flow chart was used to map out the records identified, included, and excluded, and the reasons for exclusion, Figure 1.
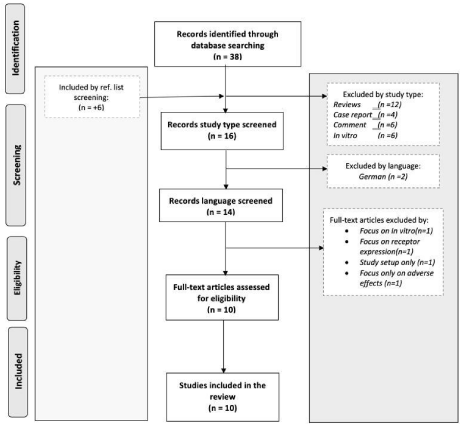
Figure 1: PRISMA flow chart.
Search strategy, inclusion and exclusion criteria
To ensure a systematic and thorough course of action, the search was based on the principles of PICO process. Searches were undertaken on Pubmed and Embase between the 1st-8th of March 2020. All clinical trials studying the application of immune checkpoint therapy in mCRPC, published in the ten years prior to March 2020, were eligible for this review. Relevant records identified through database searching were based on two search strands, differing only by the keywords related to the specific ICPI. Both search strands were based on the Medical Subject Heading database (MeSH) “Prostatic Neoplasms, Castration-Resistant” population. The strands consisted of keywords such as Ipilimumab [MeSH], CTLA4-inhibitor, PD-L1 and PD-1 inhibitor, and drug’s generic names.
General reviews, case reports, non-English records, in vitro studies, and non-ICPI trials were excluded. Endpoints of interest were Overall Survival (OS), Progression-Free Survival (PFS), clinical Overall Response Rate (ORR), and Prostate-Specific Antigen (PSA) response rate. Trials that included patients with cancer types other than PCa were excluded. Trials investigating localized or castrationsensitive PCa were also an exclusion criterion.
Data collection
The titles and abstracts of records generated by the search strands were screened against the predefined inclusion and exclusion criteria of this review. Records were also screened for duplicates. Articles whose eligibility could not be determined solely based on their titles and abstracts were selected for full-text screening. The following data was extracted from each study: study design, study phase, author names, title, objectives, patient population, mean age, performance status, treatment regimens, number of patients included, outcomes, endpoints and main findings.
Ongoing trials
A screening of ongoing studies was performed via clinicaltrials. gov using the same inclusion and exclusion criteria used for published studies.
Results and Discussion
The search strings inputted into the database generated a total of 38 records. A further 6 studies were identified through hand searching references of all 38 articles. 12 reviews, 4 case reports, 6 commentaries, 6 in vitro studies and 2 non-English studies were excluded at the titleabstract screening stage. No duplicates were identified at this stage. 14 articles were selected for full-text screening, of which 10 met the required criteria and were subsequently included in the final analysis. (Two of the included articles report on the same patient population but on different endpoints). Full details on the number of articles excluded per exclusion criteria can be found in Figure 1.
Study characteristics
The nine studies included in the final analysis consisted of two phase III trials, five phase II trials, and two phase I trials. Six trials were open label, while the remaining three trials were double blinded. Publication dates ranged from 2012 to 2019. The population sizes ranged from 10-799 patients. The mean age of the study population varied from 65 to 72 years. The follow-up period of the trials ranged from 5 to 24 months.
With regards to treatment regimens, six studies used Ipilimumab (Anti-CTLA-4), two studies used Pembrolizumab (Anti-PD-1), and one study utilized Durvalumab (Anti-PD-L1).
A wide variety of outcomes were reported, including OS, PFS, and PSA relative response. The PSA response and OS were the most frequent endpoints. Notably, not all studies used the same criteria for PSA response. Most studies considered a ≥50% drop from baseline as a PSA response, while some studies required the ≥50% drop to be met before a predefined day, e.g., ≥50% PSA drop before day 85.
The treatment regimens were similarly heterogenous, with immunotherapy used as monotherapy in three studies and used in combination with a non-immunotherapeutic drug in six studies, Figure 2.
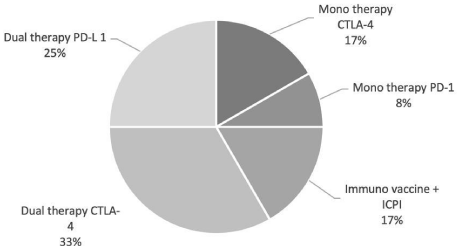
Figure 2: Included studies treatment regimens. Different regimes were used in the included studies. The figure above illustrates the incidence of different types of
regimes used in studies included in this review. Monotherapy defined as studies solely using ICPI as regime with no other interventions. Dual therapy was defined
as regimes using additional drug or treatment modality in combination with ICPI.
Even among studies testing the same therapy, drug doses and schedules were heterogeneous. Four studies experimented with dose escalation, and five with a fixed dose.
Drug administration in ipilimumab trials ranged from 0.3mg/kg every three weeks to 10 mg/kg every four weeks, with the latter being the most common.
Key study characteristics, patient demographics, and outcomes are summarized in Table 1.
Study
Patient
Mean Age
PS
Treatment type
Target
Study
Sample size
AE grade 3/4
Outcomes/endpoints
Main findings
Eertwegh et al.
and
Saskia et al. 2012mCRPC
chemo-naïve65
=1
Ipilimumab +
GVAXCTLA-4
II
28
31.00%
Median PSA decline =50%
Median OSMedian PSA decline =50%:
17.90%
Median OS:
29 msSlovin et al. 2013
mCRPC
pre-trial chemo65
=1
Ipilimumab
Ipilimumab + RTCTLA-4
I/II
Total: 71
Ipilimumab: 29
Ipilimumab + RT: 4246%
Median PSA decline =50%
Median OSMedian PSA decline =50%
Ipilimumab 25%
Ipilimumab+RT 12%
Mean OS:
17.4 msJochems et al.
2014mCRPC
pre-trial chemoIpilimumab + PROSTVAC
CTLA-4
I
30
36.60%
Median OS
Median OS:
30.3 msKwon et al, 2014
mCRPC
pre-trial chemo69
=1
RT +
Ipilimumab or
placebo
CTLA-4
III
Total: 799
Ipilimumab: 399
Placebo: 40010.1% vs. 7.3%
Median OS
Median PFS
Median PSA decline =50%Median OS
Ipilimumab:11.2ms
Placebo: 10.0 ms
PFS
Ipilimumab:
4 ms
30.7% at 6 ms
Placebo:
3.1 ms
18.1% at 6 ms
Median PSA decline =50%: Ipilimumab: 13.1%
Placebo: 5.2%Graff et al. 2016
mCRPC
6 pre-trial chemo
4 chemo-naïve72
=1
Pembrolizumab + enzalutamide
PD-1
II
10
40%
Median PSA decline =50%
Median PSA decline =50%:
42.90%Kwek et al. 2016
mCRPC
chemo-naïve70
=1
Ipilimumab +
SargramostimCTLA-4
Ib
42
26%
Median PSA decline =50%
Median OSMedian PSA decline =50%:
11.90%
OS:
23.6 msBeer et al. 2017
mCRPC
chemo-naïve70
=1
Ipilimumab vs.
PlaceboCTLA-4
III
Total: 598
Ipilimumab: 399
Placebo: 19914%
Median OS
Median PFS
Median PSA decline =50%Median OS:
Ipilimumab: 28.7 ms
Placebo: 29.7 ms
Median PFS:
Ipilim. pts: 5.6 ms
Placbo. pts: 3.8 ms
Median PSA decline >50%
Ipilim. pts: 23%
Pla.bo. pts: 8 %Karzai et al.
2018mCRPC
11 pre-trial chemo
6 chemo-naïve
66
=2
Durvalumab +
OlaparibPD-L1
II
17
82.30%
Median PSA decline >50%
Median rPFS
Median PSA decline >50%:
42.90%
rPFS at 12 ms:
51.50%
Median rPFS:
16.1 msAntonarakis et al.
2019mCRPC
pre-trial chemo
69
=2
Pembrolizumab
PD-1
II
Total: 258
Cohort 1: 133
Cohort 2: 66
Cohort 3: 5915%
Median ORR
Median DCR
Median OS
Median rPFS
Median PSA decline >50%Median ORR
Cohort 1: 5%
Cohort 2: 3%
Cohort 3: NA
Median DCR
Cohort 1: 13%
Cohort 2: 18%
Cohort 3: 39%
Median OS
Cohort 1: 9.5 ms
Cohort 2: 7.9 ms
Cohort 3:14.1 ms
Median rPFS
Cohort 1: 2.1 ms
Cohort 2: 2.1 ms
Cohort 3: 3.7 ms
Median PSA decline >50%
Coh. 1: 6.5%
Coh. 2: 8.3%
Coh. 3: 1.7%
Table 1: Study characteristics, patient demographics and outcomes from the nine studies included in this review.
PD-1 inhibitors
Antonarakis et al. [30] split 258 mCRPC patients into three cohorts; PD-L1 positive (133 patients), PD-L1 negative (66 patients), and bone predominant metastases (59 patients). PD-L1 positivity was predefined as Combined Positive Score (CPS) of ≥1. All patients were administered 200 mg Pembrolizumab. All patients had previously been treated with at least one targeted endocrine and docetaxel therapy. The data from the study demonstrated a mean OS of 9.5 months (PD-L1 positive), 7.9 months (PD-L1 negative), and 14.1 months (bone predominant metastases) and a mean PFS of 2.1, 2.1 and 3.7 months, respectively. Across all three cohorts, a decrease of PSA ≥ 50% was observed in 9% of the patients.
In contrast to Antonarakis et al., more than half of the study population (53%) in the trial by Karzai et al. [31] experienced a PSA ≥50% decrease. A total of 17 mCRPC patients, of which 65% had previously received chemotherapy, were treated with both 1500 mg Durvalumab and 300mg Olaparib (a poly ADP ribose polymerase inhibitor). All participating patients had received either Enzalutamide or Abiraterone prior to treatment. The median PFS was 16.1 months, and 51.5% of all patients were progression free at 12 months.
Graff et al. [32] showed a similar result, with PSA ≥50% in 42.8% of ten mCRPC patients (previously progressed while on Enzalutamide) treated with 200 mg Pembrolizumab combined with daily enzalutamide. At the time of follow-up (7.5 months), 30% of the patients were progression free.
Patients included in this review can be split into two broad groups; pre- and post-chemo treated mCRPC patients. A median PSA decline ≥50% was more pronounced in chemo-naïve patients, 23.94% vs. 12.2% in patients exposed previously to chemotherapy. A result that suggests that the immune depressing side effect of chemotherapy may affect the function of ICPI. A pattern was also seen in the ICPI treatment of micro satellite stable colon cancer, the respond is better if ICPI is first-line drug compared to post-chemo treated patients. However, a PSA decline ≥50% does not always reflect the antitumor effect, hence OS and PFS may be more predictive [33].
When hoping for results like the ones observed in ICPI treatment of metastatic melanoma, it is important to note that patients with mCRPC are often older patients with a weaker immune system [34]. The immune system is a key player in the ICPI treatment, hence a weakened immune system may result in a different treatment response.
Comparing the results reported in other cancer types should consequently be done with caution, especially considering how PCa differs from other cancers, in particular with regard to its endocrine controlled growth and high grade of heterogeneity. Studies even show that this heterogenicity increases with tumor progression and the number of treatment received [35]. Therefore, it may be hypothesized that a more specified patient selection should be the future for a more effective ICPI treatment in mCRPC.
CTLA-4 inhibitor
Slovin et al. [36] explored ipilimumab in 71 mCRPC patients in a two-armed trial, of which 47 discontinued due to cancer progression. Patients were allocated to either 10 mg/kg Ipilimumab monotherapy or 10 mg/kg Ipilimumab with single and focal Radiotherapy (RT). Radiation dose was of 8 Gy per target bone lesion (up to three bone lesions per patient), and was given at 24-48 h before the first ipilimumab dose. No more than one pretrial chemotherapy treatment was allowed for the patients. A PSA drop of =50% was observed in 12% of patients in the Ipilimumab/RT group compared to 25% in the Ipilimumab monotherapy group. In both arms, a median OS of 17.4 months was reported.
Kwon et al. [37] enrolled 799 patients randomized to 10 mg/kg Ipilimumab+RT (400 patients) or RT+placebo (399 patients). A single dose of bone directed radiotherapy of 8 Gy for at least one and up to five bone lesions was done some time within 2 days before initiation of the study drug regimen. All patients received at least two cycles of docetaxel in the six months prior to the start of the trial. The study demonstrated a median OS of 11.2 months in the Ipilimumab arm, versus 10.0 months in the placebo arm. Six months after treatment initiation, 30.7% of the patients treated with ipilimumab+RT were progression free versus only 18.1% of the patients in the RT+ placebo group. A PSA decline ≥50% was observed in 13.1% in the Ipilimumab+RT group and 5.2% in the RT + placebo group.
Both these studies combined ICPI with RT. Their results are comparable with less PSA decline ≥50% in the groups receiving ICPI+RT, while no significant change in OS compared to placebo was observed.
In contrast, studies show that the combination of ICPI and RT in the treatment of metastatic melanoma, non-small-cell lung cancer, and renal cell cancer enhances the immunotherapeutic effect [38]. A closer look into the RT regimes, there was an obvious dosage and fraction difference, A single fraction of 8 Gy bone directed treatment was used in Slovin et al. and Kwon et al. while 3-5 fractions with 10- 30 Gy are used in metastatic melanoma [39]. This difference suggests the need for further investigation on the effect of RT in mCRPC and researching the aim, timing, amount, and fractions of RT.
In another Ipilimumab study, Jochems et al. [40] explored dose escalation in 30 patients (24 chemo naïve) in combination with PROSTVAC immune vaccine (a T-cell modulating drug). In this study, a relatively long median OS of 30.3 months was documented. Furthermore, a positive correlation between baseline levels of circulating immature NK cells and OS was observed.
Saskia et al. [41] and Eertwegh et al. [42] studied and reported on the same study population.
Eertwegh et al. reported the initial findings and safety, while Saskia et al. reported retrospective results with a focus on OS. Both reported on a four-step dose escalation of Ipilimumab in combination with GVAX (a macrophage stimulant) that included 28 chemo-naïve mCRPC patients. No decrease in PSA ≥50% was reported in the two lowest doses, however, a decrease of PSA≥50% in 18% of the patients and a median OS of 29 months was documented in the two highest doses. Eertwegh et al. observed a significant positive correlation between PSA ≥50% decrease and longer OS.
In both of these studies (Jochems et al. and Saskia et al. Eertwegh et al.), the patients received ICPI combined with immune vaccines. These patients presented the best OS results of all included trials.
Cancer vaccines boost and reprogram the immune system to target cancer cells. This effect may just be what is needed for patients with weakened immune systems to benefit from ICPI treatment, which supports the hypothesis presented above. However, it should be taken into consideration that the two studies only reported on patient populations of 30 and 28 mCRPC patients, increasing the risk of chance findings.
In the study by Kwek et al. [43], 42 chemo and immunotherapynaïve mCRPC patients received dose-escalating Ipilimumab in combination with Sargramostim (a macrophage stimulant). A PSA ≥50% decrease in 11.9% of the patients, with a median OS of 23.6 months was observed. Results from this study demonstrated an inverse correlation between low pretreatment levels of PD-1 on T-cells and OS.
Low levels of immune system components, such as PD-1, are typically seen in immunosuppressed patients, hence this result may further emphasize the negative effects of chemotherapy prior to ICPI treatment.
Beer et al., [44] compared a treatment regimen of 10mg/kg Ipilimumab (399 patients) with placebo (199 patients) in chemo naïve patients. The authors found no significant difference in median OS between the Ipilimumab group (28.7 months) and the placebo group (29.7 months). However, treatment with ipilimumab was associated with both longer PFS and longer median PSA decline ≥50% in comparison to the placebo group.
Safety
As ICPI’s are still a relatively new treatment, and many trials included in this review sought to report on safety outcomes such as treatment-related Adverse Events (AE). The rate of grade 3 and 4 side effects in the included studies ranged from 10.1 to 82.3 %. In six studies, the rate of these side effects ranged between 25% and 60 %. The relatively high incidence of AE may be linked to the high proportion of patients who received chemotherapy prior to the study, as >60% of patients across the included studies had received chemotherapy prior to trial start. A well-known side effect of chemotherapy is immunosuppression, which could possibly explain why patients were more vulnerable to AE on immunotherapy. When studies are characterized into a CTLA-4 group and a PD-1/PDL-1 group, 44% of patients in the PD-1/PDL-1 trials and only 27.2% in the CTLA-4 trials experienced grade 3 or 4 AE. However, the percentage of patients discontinuing treatment based on AE in CTLA-4 trials, was 27% and 4.5% in PD-1/PDL-1, emphasizing that AE’s caused by CTLA-4 are more severe leading to greater rates of discontinuation.
A similarly noteworthy difference was observed with regard to the drug dosage used. In the CTLA-4 studies, drugs were administered per kg body weight while in PD-1/PDL-1 studies a fixed dose was administered regardless of the patient’s volume of distribution. This may result in patients reaching either over or under the therapeutic window, risking either severe side-effects or no therapeutic effect.
A practical example of this presents itself in Kwon et al. [37] and Beer et al. [44], the two largest CTLA-4 studies in the review. Both studies contained an ipilimumab group and a placebo group. Both studies reported higher AE’s in the Ipilimumab group.
In Kwon et al., the rate of AE’s was 10.1% in the Ipilimumab group compared to 7.3% in the control group, while Beer et al. reported 56% and 30% respectively.
Discontinuation due to AE’s was also higher in the ipilimumab group in both studies. In Beer et al., 29% of patients were discontinued in the Ipilimumab and only 3% in the placebo group. Similarly, 35% of the patients in the Ipilimumab group discontinued due to AE’s in the Kwon et al. study compared to 16% in placebo group. This difference in the two groups emphasizes the potential toxicity, most probably due to the narrow therapeutic window of ipilimumab.
The type of side effects observed across the different studies are relatively homogenous, as opposed to the proportion of patients experiencing side effects. Grade 3 and 4 diarrheas were reported in eight out of nine trials. This comes as no surprise as diarrhea is a wellknown side effect of ICPI [45]. Rash, fatigue, and anemia also top the list of grade 3 and 4 side effects, however, there was no evidence of a link between these side effects and specific drug and treatment regimes.
Ongoing trials and the future of mCRPC
Numerous ICPI studies are ongoing, making it relevant to summon and describe the study characteristics. These ongoing trials share many characteristics with the studies covered in this review, such as the stratifying of patients based on Tumor Mutation Burden (TMB) and expression of relevant biomarkers/receptors. When comparing ongoing mCRPC studies to completed mCRPC studies, it is clear that this stratifying plays a more significant role in the ongoing studies. Completed studies predominantly listed safety outcomes as their primary outcome, whereas ongoing studies have put greater emphasis on treatment efficiency, with safety as a secondary endpoint.
A frequent pattern that is observed in ongoing studies, but not in completed studies, is the experimentation with a combination of two ICPI drugs. The choice of study drug has also changed over the years, with a domination of CTLA-4 drugs (like ipilimumab) in completed studies, while ongoing trials are predominantly exploring PD-1/ PD-L1 drugs. Among the ongoing studies, only 12 use CTLA-4 ICPI whereas 40 use PD-L/PD-L1 ICPI, with Pembrolizumab being used in 20 of the 40 trials, Figure 3.
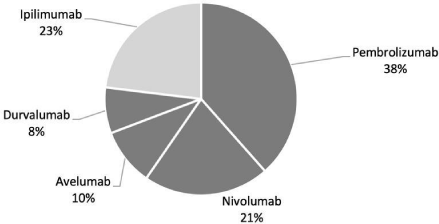
Figure 3: Drug regimens used in ongoing trials. The figure illustrates the frequency of drugs used in the ongoing trials found on clinicaltrials.gov. Light grey part
illustrates CTLA-4 ICPI, dark grey parts illustrates PDL-1/PDL-1 ICPI.
The combinational approaches have and will dominate the ICPI research in mCRPC over the forthcoming decade. The combination of two ICPI’s in one study population can be observed in many ongoing trials, a new pattern of combination aspiring for great results.
Strength and Limitations
This review provides an up-to--to-date overview of the current role of immunotherapy in the treatment of mCRPC. Articles published in the last ten years were included following an extensive, systematic search of Pubmed and Embase databases. This was supplemented with hand search of reference lists of relevant articles. However, this review has a number of limitations.
First of all, only two biomedical databases were searches due to time constraints and this may have led to missing articles published through other relevant databases. Moreover, the search was limited to English language articles, thereby excluding articles published in other languages. A further important limitation is the inclusion of only published articles. The grey literature was not searched for unpublished trials. Published trials are prone to publication bias [46].
Finally, no quality appraisal of the included studies was undertaken and only a single author conducted the screening.
Conclusion
Immunotherapy role in mCRPC is still in its infancy, although the preliminary studies show promising results. Further research is still awaiting to thoroughly understand prostate tumor immunobiology as well as continue the search for the perfect combinational treatment. Whether a one-for-all personalized combination with checkpoint inhibitors or the incorporation of modalities such as cancer vaccines or RT, a successful treatment will most likely have to be a multibranched approach. While it is inspiring to see a rising amount of ICPI trials in PCa over the last decade, the lack of conclusive data on significant treatment responses stands tall as a clear reminder of the long road ahead in the field of mCRPC.
References
- Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015; 136: E359-E386.
- Patrikidou A, Loriot Y, Eymard JC, et al. Who dies from prostate cancer? Prostate Cancer Prostatic Dis. 2014; 17: 348-352.
- Vinay DS, Kwon BS. Harnessing immune checkpoints for cancer therapy. Immunotherapy. 2018; 10: 1265–1285.
- Waldman AD, Fritz JM, Lenardo MJ. A guide to cancer immunotherapy: from T cell basic science to clinical practice. Nature Reviews Immunology. 2020; 20: 651-668.
- Hodi FS, O’Day SJ, McDermott DF, et al. Improved Survival with Ipilimumab in Patients with Metastatic Melanoma. N Engl J Med. 2010; 363: 711-723.
- Shafer-Weaver KA, Anderson MJ, Stagliano K, et al. Cutting Edge: Tumor- Specific CD8+ T Cells Infiltrating Prostatic Tumors Are Induced to Become Suppressor Cells. J Immunol. 2009; 183: 4848-4852.
- Kantoff PW, Higano CS, Shore ND, et al. Sipuleucel-T Immunotherapy for Castration-Resistant Prostate Cancer. N Engl J Med. 2010; 363: 411-422.
- Walker LSK. Treg and CTLA-4: Two intertwining pathways to immune tolerance. Journal of Autoimmunity. 2013; 45: 49-57.
- Bour-Jordan H, Esensten JH, Martinez-Llordella M, et al. Intrinsic and extrinsic control of peripheral T-cell tolerance by costimulatory molecules of the CD28/B7 family. Immunological Reviews. 2011; 241: 180-205.
- Lenschow DJ, Walunas TL, Bluestone JA. CD28/B7 system of T cell costimulation. Annual Review of Immunology. 1996; 14: 233-258.
- Sansom DM. CD28, CTLA-4 and their ligands: Who does what and to whom? Immunology. 2000; 101: 169-177.
- Linsley PS, Bradshaw J, Greene JA, et al. Intracellular trafficking of CTLA-4 and focal localization towards sites of TCR engagement. Immunity. 1996; 4: 535-543.
- Wolchok JD, Saenger Y. The Mechanism of Anti-CTLA-4 Activity and the Negative Regulation of T-Cell Activation. Oncologist. 2008; 13: 2-9.
- Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade. Science (80-). 1996; 271: 1734-1736.
- Peggs KS, Quezada SA, Korman AJ, et al. Principles and use of anti-CTLA4 antibody in human cancer immunotherapy. Current Opinion in Immunology. 2006; 18: 206-213.
- Buchbinder EI, Desai A. CTLA-4 and PD-1 pathways similarities, differences, and implications of their inhibition. American Journal of Clinical Oncology: Cancer Clinical Trials. 2016; 39: 98-106.
- Seidel JA, Otsuka A, Kabashima K. Anti-PD-1 and anti-CTLA-4 therapies in cancer: Mechanisms of action, efficacy, and limitations. Frontiers in Oncology. 2018.
- Ceeraz S, Nowak EC, Noelle RJ. B7 family checkpoint regulators in immune regulation and disease. Trends in Immunology. 2013; 34: 556-563.
- Keir ME, Butte MJ, Freeman GJ, et al. PD-1 and its ligands in tolerance and immunity. Annual Review of Immunology. 2008; 26: 677-704.
- Francisco LM, Salinas VH, Brown KE, et al. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J Exp Med. 2009; 206: 3015-3029.
- Kinter AL, Godbout EJ, McNally JP, et al. The Common γ-Chain Cytokines IL-2, IL-7, IL-15, and IL-21 Induce the Expression of Programmed Death-1 and Its Ligands. J Immunol. 2008; 181: 6738-6746.
- Riley JL. PD-1 signaling in primary T cells. Immunological Reviews. 2009; 229: 114-125.
- Iwai Y, Ishida M, Tanaka Y, et al. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc Natl Acad Sci USA. 2002; 99: 12293-12297.
- Isaacsson Velho P, Antonarakis ES. PD-1/PD-L1 pathway inhibitors in advanced prostate cancer. Expert Rev Clin Pharmacol. 2018; 11: 475-486.
- Alsaab HO, Sau S, Alzhrani R, et al. PD-1 and PD-L1 checkpoint signaling inhibition for cancer immunotherapy: mechanism, combinations, and clinical outcome. Frontiers in Pharmacology. 2017; 8: 561.
- Dotti G. Blocking PD-1 in cancer immunotherapy. Blood. 2009; 114: 1457- 1458.
- Gong J, Chehrazi-Raffle A, Reddi S, et al. Development of PD-1 and PD-L1 inhibitors as a form of cancer immunotherapy: A comprehensive review of registration trials and future considerations. Journal for ImmunoTherapy of Cancer. 2018; 6: 8.
- Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. In: Journal of clinical epidemiology. J Clin Epidemiol. 2020; e1-e34.
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Int J Surg. 2010; 8: 336-341.
- Antonarakis ES, Piulats JM, Gross-Goupil M, et al. Pembrolizumab for Treatment-Refractory Metastatic Castration-Resistant Prostate Cancer: Multicohort, Open-Label Phase II KEYNOTE-199 Study. American Society of Clinical Oncology. 2020.
- Karzai F, Vanderweele D, Madan RA, et al. Activity of durvalumab plus olaparib in metastatic castration-resistant prostate cancer in men with and without DNA damage repair mutations 11 Medical and Health Sciences 1112 Oncology and Carcinogenesis. Journal for ImmunoTherapy of Cancer. 2018.
- Graff JN, Alumkal JJ, Drake CG, et al. Early evidence of anti-PD-1 activity in enzalutamide-resistant prostate cancer. Oncotarget. 2016; 7: 52810-52817.
- Rathkopf DE, Beer TM, Loriot Y, et al. Radiographic Progression-Free Survival as a Clinically Meaningful End Point in Metastatic Castration- Resistant Prostate Cancer. JAMA Oncol. 2018; 10065: 1-8.
- Montecino-Rodriguez E, Berent-Maoz B, Dorshkind K. Causes, consequences, and reversal of immune system aging. Journal of Clinical Investigation. 2013; 123: 958-965.
- Massard C, Oulhen M, Le Moulec S, et al. Phenotypic and genetic heterogeneity of tumor tissue and circulating tumor cells in patients with metastatic castrationresistant prostate cancer: A report from the PETRUS prospective study. Oncotarget. 2016; 7: 55069-55082.
- Slovin SF, Higano CS, Hamid O, et al. Ipilimumab alone or in combination with radiotherapy in metastatic castration-resistant prostate cancer: Results from an open-label, multicenter phase i/ii study. Ann Oncol. 2013; 24: 1813- 1821.
- Kwon ED, Drake CG, Scher HI, et al. Ipilimumab versus placebo after radiotherapy in patients with metastatic castration-resistant prostate cancer that had progressed after docetaxel chemotherapy (CA184-043): A multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol. 2014; 15: 700-712.
- Van Limbergen EJ, De Ruysscher DK, Pimentel VO, et al. Combining radiotherapy with immunotherapy: The past, the present and the future. British Journal of Radiology. 2017.
- Escorcia FE, Postow MA, Barker CA. Radiotherapy and Immune Checkpoint Blockade for Melanoma: A Promising Combinatorial Strategy in Need of Further Investigation. Cancer Journal (United States). 2017; 23: 32-39.
- Jochems C, Tucker JA, Tsang KY, et al. A combination trial of vaccine plus ipilimumab in metastatic castration-resistant prostate cancer patients: Immune correlates. Cancer Immunol Immunother. 2014; 63: 407-418.
- Santegoets SJAM, Stam AGM, Lougheed SM, et al. T cell profiling reveals high CD4+CTLA-4+ T cell frequency as dominant predictor for survival after Prostate GVAX/ipilimumab treatment. Cancer Immunol Immunother. 2013; 62: 245-256.
- M van den Eertwegh AJ, Versluis J, Pieter van den Berg H, et al. Combined immunotherapy with granulocyte-macrophage colony-stimulating factortransduced allogeneic prostate cancer cells and ipilimumab in patients with metastatic castration-resistant prostate cancer: A phase 1 dose-escalation trial. Lancet Oncol. 2012; 13: 509-517.
- Kwek SS, Lewis J, Zhang L, et al. Preexisting levels of CD4 T cells expressing PD-1 are related to overall survival in prostate cancer patients treated with ipilimumab. Cancer Immunol Res. 2015; 3: 1008-1016.
- Beer TM, Kwon ED, Drake CG, et al. Randomized, double-blind, phase III trial of ipilimumab versus placebo in asymptomatic or minimally symptomatic patients with metastatic chemotherapy-naive castration-resistant prostate cancer. J Clin Oncol. 2017; 35: 40-47.
- Wang Y, Abu-Sbeih H, Mao E, et al. Immune-checkpoint inhibitor-induced diarrhea and colitis in patients with advanced malignancies: Retrospective review at MD Anderson. J Immunother Cancer. 2018.
- Murad MH, Chu H, Lin L, et al. The effect of publication bias magnitude and direction on the certainty in evidence. BMJ evidence-based Med. 2018; 23: 84-86.