
Case Report
Austin J Clin Case Rep. 2022; 9(2): 1244.
Resection and Reconstruction of Fibrocartilaginous Mesenchymoma of the Distal Fibula: A Case Report and Review of the Literature
Jiang L, Niu K, Ma C, Hanlin X, Wnag and Gao S*
Henan University People’s Hospital, Henan Provincial People’s Hospital, China
*Corresponding author: Songtao Gao, Henan Provincial People’s Hospital, China
Received: February 17, 2022; Accepted: March 11, 2022; Published: March 18, 2022
Abstract
Fibrocartilaginous mesenchymoma (FM) is a rare, locally aggressive bone tumor, with only 37 cases have been reported in the literature since 1984. This tumor principally occurs in long metaphysis bones in children and adolescents. In this case, a 13-years-old boy presented to the hospital with a firm, immobile, painful and slow growing mass of the right ankle that had been apparent for 4 months. The pathology after the biopsy showed Fibrocartilaginous mesenchymoma. We performed right fibula distal tumor resection and vascularized fibula reverse graft to repair the bone defect, which not only completely resected the tumor, but also preserved the stability of the ankle joint and ensured blood supply of the fibula reverse graft. The ankle joint function was stable in the short-term follow-up after operation.
Keywords: Fibrocartilaginous mesenchymoma; Tumor of the distal fibula; Bone tumor; Vascularized fibular graft; Resection; Reconstruction
Introduction
Fibrocartilaginous mesenchymoma (FM) is a rare, locally aggressive, primary intraosseous borderline tumor with unknown etiology, all of which are single, often asymptomatic or only manifested as pain or swelling at the lesion site [1]. Radiologically, FM appears as an expansible osteolytic lesion with cartilaginous calcification and cortical destruction, and extension to soft tissue is not uncommon. Histologically, FM is characterized by spindle cell proliferation in association with bland cartilage nodules and epiphyseal growth platelike enchondral ossification [2].
Fibrocartilaginous mesenchymoma was first reported by Dahlin et al. in 1984 [3]. A total of 37 cases of FM have been reported in foreign literature (Table 1). In all reports, patients range in age from three months to 27 years (median age of 13 years) at the onset of symptoms. Among them, there are 25 males and 12 females (male: Female=2.08:1.00), this tumor occurs in long metaphysis bones (21 cases), other locations are the iliac-pubic bone (6 cases), vertebrae (6 cases), ribs (2 cases), and metatarsal (1 case). All cases were followed up for 4 to 196 months, and 4 cases relapsed after partial resection. In this case, the patient was followed up for 9 months after the operation, and there was no sign of recurrence.
Patient and Reference
Age (years), Sex
Treatment
Further treatment
Follow up
1 [4,5]
10y F
Wide resection
–
NED at 4y
2 [4,5]
9y M
First metatarsal
Intralesional excision
Wide contaminated resection of recurrence (1y later); then wide resection of a second recurrence (1y later)
NED at 12y
3 [4,5]
14y M
Intralesional excision
Wide resection of recurrence (6y later)
NED at 78mo
4 [4,5]
9y M
Metaphysis proximal fibula
Wide resection
–
NED at 5y
5 [4,5]
22y M
L4
Intralesional excision
–
NED at 10y
6 [4,5]
14y M
Diaphysis fibula
Intralesional biopsy
Intralesional biopsy (1y later)
UK
7 [4,5]
14y M
Proximal tibia
Resection
–
NED at 5y
8 [4,5]
15y F
Pubis
UK
–
UK
9 [4,5]
25y F
Proximal humerus
Resection
NED at 2y
10 [4,5]
16y M
Proximal tibia
Intralesional excision
Resection of recurrence (2y later)
NED at 2y
11 [4,5]
12y F
Proximal Fibula
Resection
NED at 1y
12 [5,16]
11y M
Iliac bone
Incomplete excision, then wide resection
–
NED at 14y
13 [5,17]
4y M
Metaphysis proximal humerus
Curettage with adjuvant penalization
–
14 [5,18]
17y F
Intralesional excision with phenol and ethanol cauterization
NED at 1y
15 [5,19]
3mo F
Metaphysis proximal tibia
complete regression after 14mo
16 [5,20]
11y M
Metaphysis proximal humerus
Forequarter amputation
NED 2y
17 [5,21]
9y M
T12
–
NED 2y
18 [5,22]
15y F
Metaphysis distal femur
Resection and curettage of a part of the tumor
–
NED 5y
19 [5,23]
9y M
Wide resection
NED 4y
20 [5,24]
9y M
Ilium
UK
21 [5,25]
14y M
Metaphysis proximal humerus
Resection
–
UK
22 [5,26]
19y M
L5
Wide contaminated resection
NED 5y
23 [5,27]
12y M
Metaphysis proximal tibia
Curettage
Wide resection of recurrence (1y later)
NED 2y
24 [5,27]
1y 7mo M
Metaphysis proximal tibia
Curettage
–
Decreased in size after 4mo
25 [5,28]
11y M
Metaphysis proximal tibia
Curettage, then wide resection
NED 10y
26 [5]
8y F
Metaphysis proximal femur
UK
27 [5]
10y M
Distal femur
Intralesional excision
UK treatment of recurrence (1y later)
UK
28 [5]
27y F
Pubis
Resection
29 [5]
18y F
Ilium-pubis
Resection and curettage of a part of the tumor
–
NED 65mo
30 [5]
18y F
L3
En bloc wide resection
NED 10y
31 [5]
13y M
L4
En bloc wide resection
32 [5]
14y M
Intercalary resection
–
UK
33 [5]
22y F
Metaphysis proximal humerus
Curettage
wide Tikhoff-Linberg resection (2 weeks later)
34 [1]
13y M
L3
Wide resection
–
NED 9y
35 [29]
16y M
Metaphysis proximal tibia
En bloc resection
–
NED 7mo
36 [2]
17y M
Fifth rib
En bloc resection
–
NED 1y
37cs
13y M
Distal fibula
En bloc resection
–
NED 6mo
Legend: y: year(s); mo: months; UK: Unknown; M: Male; F: Female; NED: Not Evidence of Disease; cs: current study.
Table 1: Location, clinical features, further treatment, and follow-up.
Case Presentation
A 13-years-old boy presented to Henan Provincial People’s Hospital (Henan, China) with a firm, immobile, painful and slow growing mass of the right ankle that had been apparent for 4 months, the pain was aggravated during activity. A physical examination revealed there was a long oval mass on the right ankle, which was firm, immobile, painful, no local redness and local skin temperature was not high. Laboratory examination of alkaline phosphatase (ALP: 466.0 U/L) was higher than the normal value. X-ray (Figure 1A) revealed: a cystic low-density shadow could be seen at the distal end of the right fibula, with a clear boundary, no obvious sclerosis zone around it, and swelling growth. There was no obvious abnormality in the adjacent bone marrow cavity, and the local bone cortex was thinning and defect; multiple atrial septals were seen in the lesion, and no obvious periosteal reaction was seen. Computed tomography (CT) (Figure 1B) revealed that the distal end of the right fibula showed swelling changes, with patchy dense calcifications and cysts, and soft tissue density shadows, and the lesions did not cross the epiphyseal line. Magnetic resonance imaging (MRI) (Figure 1C) revealed that the swelling changes in the distal metaphysis of the right fibula showed long T1 and long T2 signal shadows, and the boundary was clear. Line-like long T2 signal shadows were visible in the surrounding soft tissues, and there was no more obviously abnormal. Emission CT (ECT) (Figure 1D) revealed that only the radioactive abnormal concentration was seen in the distal end of the right fibula, and there was no obvious abnormality in bone metabolism in the rest. The pathology of needle biopsy under local anesthesia showed that the possibility of fibrocartilaginous mesenchymoma was considered. It was recommended to confirm the diagnosis after tumor resection.
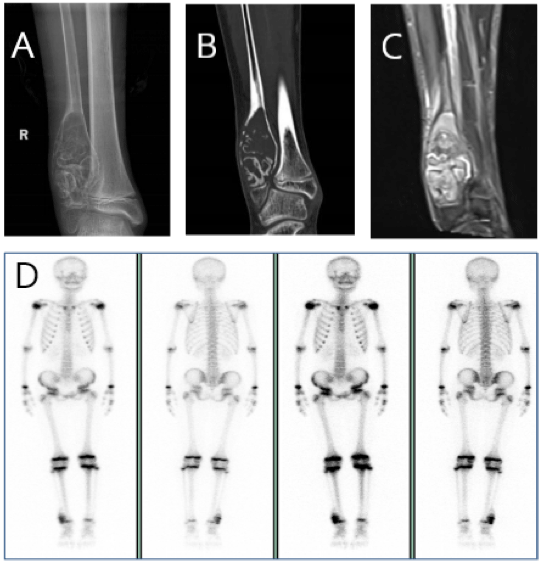
Figure 1: A) X-ray revealed that a cystic low-density shadow could be seen at the distal end of the right fibula, with a clear boundary. B) CT revealed that the
distal end of the right fibula showed swelling changes, with patchy dense calcifications and cysts, and soft tissue density shadows, and the lesions did not cross
the epiphyseal line. C) MRI revealed that the swelling changes in the distal metaphysis of the right fibula showed long T1 and long T2 signal shadows, and the
boundary was clear. Line-like long T2 signal shadows were visible in the surrounding soft tissues, and there was no more Obviously abnormal. D) ECT revealed
that only the radioactive abnormal concentration was seen in the distal end of the right fibula, and there was no obvious abnormality in bone metabolism in the rest.
Operative steps
The tumor was approached through a direct posterolateral incision over the distal half of fibula. The cutaneous branch of superficial peroneal nerve was identified and preserved. The distal end of the tumor did not invade the epiphysis, the proximal end of the tumor was osteotomized along the epiphyseal line, the epiphysis was preserved (about 1cm), the proximal end of the tumor 3 cm outside was osteotomized, and the tumor was completely resected (Figure 2A). In order to ensure the blood supply of the fibula reverse graft, we found a nourishing artery (distal branch of the peroneal artery) at the normal fibula near the fractured end of the osteotomy, and measured the distance about 5cm from the distal branch of the peroneal artery to the distal epiphyseal line of the fibula epiphysis, and osteotomized the normal fibula from the distal branch of the peroneal artery to the proximal end of the normal fibula at about 5cm and dissected the fibula periosteum. The vascularized fibular graft was inverted, and we reconstructed the proximal fibula osteotomy surface and the distal epiphysis osteotomy surface (Figure 2B). We reconstructed the inverted vascularized fibular graft with a titanium plate and screws. X-rays showed that the internal fixation was firm and ankle joint was stabilized (Figure 2C).
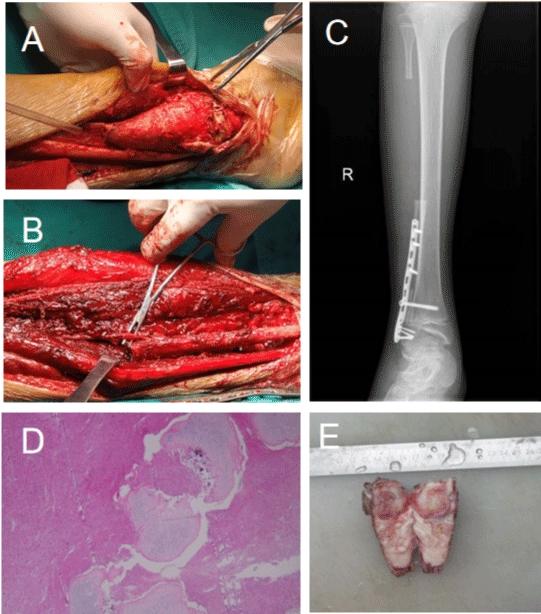
Figure 2: A) Exposing and removing the tumor of distal fibula. B) Inverting the vascularized fibular graft and reconstructing the proximal fibula osteotomy surface
and the distal epiphysis osteotomy surface. C) Reconstructing the inverted vascularized fibular graft with a titanium plate and screws. X-ray examination showing
that the internal fixation was firm and the ankle joint was stable. D) Pathological photomicrographs showed a large number of spindle cell proliferations between
well-differentiated cartilage islands, mildly atypia of tumor cells, occasional mitotic images, no tumor necrosis, and focal cartilage matrix calcification and ossification.
Scattered multinucleated giant cells can be seen, and some trabecular bones are seen around. E) The pathology is generally gray and white matter or fibrous
tissue, with translucent and brittle cartilage nodules scattered in the middle.
The ankle was immobilized in a below knee back slab till suture removal. At suture removal, an ankle brace was given and patient was allowed intermittent ankle ROM as tolerated and touches weight bearing with bilateral axillary crutches for 6 weeks progressing to full weight bearing on ankle brace for another 6 weeks. Till 4 months, he was on protected weight bearing with ankle brace. Clinical and radiological follow up was done regularly. At 9 months follow up, clinical assessment was done with American Orthopaedic Foot and Ankle Society score and radiological assessment. At follow up at 9 months, there was no ankle instability. The AOFAS score was excellent (92/100). His dorsi flexion was 20°and plantar flexion was 40°.
The pathology (Figure 2E) is generally gray and white matter or fibrous tissue with tough texture, and there are scattered and distributed translucent and brittle cartilage nodules. The photomicrograph image (Figure 2D) shows that a large number of spindle cell proliferations are seen between the well-differentiated cartilage islands, the tumor cells are slightly atypia, mitotic images are occasionally (0-1/10HPF), and no tumor necrosis is seen. Localized cartilage matrix calcification and ossification, scattered multinucleated giant cells, and part of bone trabecula were seen around. Combined with immunohistochemical markers, it was consistent with fibrocartilage mesenchymal tumor; immunohistochemical results showed: -A1:B-Catenin (-), Bcl-2 (scattered +), Bcl-6 (-), CD31 (-), CD34 (-), CDK4 (-), CK (AE1/AE3) (-), CK19 (-), CK7 (-), Desmin (-), Ki67 (5%+), MDM2 (+), MSA (-), MyoD1 (-), Myogenin (-), Napsin A (-), Nestin (-), P16 (+), P53 (-), S-100 (cartilage island +), SMA (-), STAT6 (-), Villin (-), Factor VIII (vessel +), H3F3AG34W (-), SATB2 (+). FISH result: It is indicated that the MDM2 gene of the sample submitted for inspection was not amplified (negative). IDH: The IDH1/IDH2 gene mutation test result of the sample submitted for inspection is no mutation (wild type).
Discussion
Fibrocartilage mesenchymal tumor is a rare primary intraosseous borderline tumor [1]. In 1984, Dahlin et al. reported 5 cases of FM for the first time. Among them, 3 cases of local recurrence were named “low-grade malignant tumors” [3]. In 1993, Bulychova et al. retrospectively analyzed the case data of 12 FM patients and believed that incomplete resection could lead to local tumor recurrence, but no metastasis or death, so it could not be classified as “low-grade malignancy”. “Follow-up case analysis and follow-up showed that there were few recurrences and metastases after complete resection of the lesions, which further proved this view [4]. The 5th edition of the World Health Organization classification of bone tumors newly listed FM as an independent tumor entity.
Radiologically, FM appears as an expansile osteolytic lesion with cartilaginous calcification and cortical destruction, and extension to soft tissue is not uncommon. Histologically, FM is characterized by spindle cell proliferation in association with bland cartilage nodules and epiphyseal growth plate-like enchondral ossification. The differential diagnoses include FCD (fibrocartilaginous dysplasia, FCD), low-grade osteosarcoma, dedifferentiated chondrosarcoma, desmoplastic fibroma, and chondromesenchymal hamartoma of the chest wall [2]. In 2017, Gambarotti M, et al. [5] analyzed eight new cases from the files of the Istituto Ortopedico Rizzoli dating from 1982 to 2016. This very rare bone tumor has a typical radiological and histological pattern and a favorable survival outcome after treatment. Local recurrences can be prevented with complete surgery. FM does not seem to be genetically related to fibrous dysplasia, low-grade osteosarcoma, and dedifferentiated chondrosarcoma.
Tumors of the distal fibula are rare. However, their management poses significant challenges. Given the low incidence and diverse nature of distal fibular tumors, there are several solutions [6]. For instance, distal fibular resection without reconstruction of the lateral side of the ankle is frequently performed [7]. In such instances, ankle stability is obtained via either soft tissue and ligament reconstruction or tibiotalar arthrodesis. In other cases, fibular resection is followed by reconstruction with allograft, autografts, pedicled vascularized epiphyseal transfers using the ipsilateral proximal fibula or a long bone graft from the iliac crest, bone transplants, or prosthetic ankle joint replacement [8-10].
Different techniques for reconstruction of the distal fibula after wide tumor resection have been described. All methods have different advantages and disadvantages [11]. In this case, the tumor did not attack the distal fibula epiphysis and epiphysis line, and the child’s epiphysis was not closed. If the distal fibula bone was completely resected directly, the loss of distal epiphysis would not only lose the opportunity of joint development, but also cause the loss of ankle joint stability. Resection of the lateral ankle can cause varus instability or a collapse into valgus [12]. If the distal fibula is resected and reconstructed with ipsilateral fibulae capitulum, loss of the proximal fibula can cause lateral knee instability or a damage of the peroneal nerve [13-15]. Another disadvantage of this technique is the incongruity of the fibula head with the articulating talus and the risk of pseudarthrosis. In addition, the distal branch of the peroneal artery which could be used as an effective blood supply source for the fibular graft ensured the blood supply of the inverted fibular graft and avoided the undesirable healing of the broken end of the fibular graft and the distal epiphysis of the fibular graft due to poor blood supply.
In summary, bone FM is a rare intraosseous borderline primary tumor characterized by fibrous hyperplasia and epiphyseal cartilage formation. FM is a locally aggressive tumor with no distant metastasis reported thus far. Local recurrence occurs only in cases of incomplete removal, such as curettage or intralesional excision. Complete surgical excision with adequate margins is the treatment of choice for FM.
References
- Dong RF, Li L, Su YB, Zhang M, Sun XQ, Ding Y. Fibrocartilaginous mesenchymoma: report of a case. Zhonghua Bing Li Xue Za Zhi. 2021; 50: 63-65.
- Oh SJ. Fibrocartilaginous mesenchymoma with an unusual location in the rib. J Pathol Transl Med. 2021; 55: 75-78.
- Dahlin D, Bertoni F, Beabout J, Campanacci M. Fibrocartilaginous mesenchymoma with low-grade malignancy. Skeletal radiology. 1984; 12: 263-269.
- Bulychova I, Unni K, Bertoni F, Beabout J. Fibrocartilagenous mesenchymoma of bone. The American journal of surgical pathology. 1993; 17: 830-836.
- Gambarotti M, Righi A, Vanel D, Cocchi S, Benini S, Elli F, et al. Fibrocartilaginous mesenchymoma of bone: a single-institution experience with molecular investigations and a review of the literature. Histopathology. 2017; 71: 134-142.
- Perisano C, Marzetti E, Spinelli M, Graci C, Fabbriciani C, Maffulli N, et al. Clinical management and surgical treatment of distal fibular tumours: a case series and review of the literature. International orthopaedics. 2012; 36: 1907- 1913.
- Mohler D, Cunningham D. Adamantinoma arising in the distal fibula treated with distal fibulectomy: a case report and review of the literature. Foot & ankle international. 1997; 18: 746-751.
- Lee S, Kim H, Park Y, Rhie T, Lee H. Prosthetic reconstruction for tumours of the distal tibia and fibula. The Journal of bone and joint surgery British volume. 1999; 81: 803-807.
- Eger W, Schörle C, Zeiler G. Giant cell tumor of the distal fibula: fifteenyear result after en bloc resection and fibula reconstruction. Archives of orthopaedic and trauma surgery. 2004; 124: 56-59.
- de Gauzy J, Kany J, Cahuzac J. Distal fibular reconstruction with pedicled vascularized fibular head graft: a case report. Journal of pediatric orthopedics Part B. 2002; 11: 176-180.
- Dieckmann R, Ahrens H, Streitbürger A, Budny T, Henrichs M, Vieth V, et al. Reconstruction after wide resection of the entire distal fibula in malignant bone tumours. International orthopaedics. 2011; 35: 87-92.
- Jones R, Ishikawa S, Richardson E, Murphy G. Effect of distal fibular resection on ankle laxity. Foot & ankle international. 2001; 22: 590-593.
- Leibner E, Ad-El D, Liebergall M, Ofiram E, London E, Peyser A. Lateral malleolar reconstruction after distal fibular resection. A case report. The Journal of bone and joint surgery American. 2005; 87: 878-882.
- Bickels J, Kollender Y, Pritsch T, Meller I, Malawer M. Knee stability after resection of the proximal fibula. Clinical orthopaedics and related research. 2007; 454: 198-201.
- Erler K, Demiralp B, Ozdemir M, Basbozkurt M. Treatment of proximal fibular tumors with en bloc resection. The Knee. 2004; 11: 489-496.
- Cozzutto C, Cornaglia-Ferraris P. Fibrocartilaginous mesenchymoma of bone. Pathology, research and practice. 1991; 187: 279-283.
- Masquijo JJ, Sartori F, Innocenti S. Fibrocartilaginous mesenchymoma of the proximal humerus: case report. Arch Argent Pediatr. 2014; 112: e222-226.
- Takahashi Y, Oda Y, Yamamoto H, Ishii T, Setsu N, Endo M, et al. Fibrocartilaginous mesenchymoma arising in the pubic bone: a case report. Pathol Int. 2013; 63: 226-229.
- Kumar V, Behera P, Shashikanth VS, Sudesh P. Congenital mesenchymoma of tibia: case report and review of literature. J Pediatr Surg. 2012; 47: e17-20.
- Lin J, Shulman SC, Steelman CK, Oskouei SV, Reith JD, Simoneaux SF, et al. Fibrocartilaginous mesenchymoma, a unique osseous lesion: case report with review of the literature. Skeletal Radiol. 2011; 40: 1495-1499.
- Martinez-Lage JF, Alarcon F, Hernandez-Barcelo JE, Almagro MJ, Alfaro R, Galera-Minarro A. Fibrocartilaginous mesenchymoma of the spine in a child: a case report. Childs Nerv Syst. 2010; 26: 385-389.
- Hatori M, Watanabe M, Okada K, Hosaka M, Kokubun S. Fibrocartilaginous mesenchymoma arising in the femur. Pathology. 2002; 34: 199-201.
- Gedikoglu G, Aksoy M, Ruacan S. Fibrocartilaginous mesenchymoma of the distal femur: case report and literature review. Pathology international. 2001; 51: 638-642.
- Sumner T, Ward W, Kilpatrick S, Opatowsky M. Fibrocartilaginous mesenchymoma of bone: case report and review of the literature. Pediatric radiology. 2000; 30: 315-317.
- Cherradi N, Jelthi A, Alhamany Z, Miri A, Forest M. Fibrocartilaginous mesenchymoma of bone. A case report. Clinical and experimental pathology. 1999; 47: 249-255.
- Gibson J, Reid R, McMaster M. Fibrocartilaginous mesenchymoma of the fifth lumbar vertebra treated by vertebrectomy. Spine. 1994; 19: 1992-1997.
- Hayes S, Wells S, Harake J, Henderson J, Malcolm A. Fibrocartilagenous mesenchymoma of bone: the youngest reported case in a patient aged 1 year and 7 months. Journal of clinical pathology. 2005; 58: 782-783.
- MJ Sangüesa Nebot JGL, C Valverde Mordt. Fibrocartilaginous mesenchymoma of bone. Case report. Revista Española de Cirugía Ortopédica y Traumatología. 2007; 41: 30-33.
- Saito T, Motoi T, Suehara Y, Takagi T, Okubo T, Kurihara T, et al. Fibrocartilaginous mesenchymoma of the tibia with predominant microcystic features: A case report and literature review. Human Pathology: Case Reports. 2019; 16.