
Retrospective Study
Austin J Clin Case Rep. 2022; 9(4): 1255.
Isolated Dilation of Right Heart Chambers in Fetal Echocardiography: Correlation with Postnatal Findings in a Quarterly Hospital
Gallafrio CC*, Lianza AC, Afonso TR, Sawamura KSS, Rodrigues ACT, Tavares GMP, Sanchez RC, Oliveira WAA, Vieira MLC, Fischer CH and Morhy SS
Department of Echocardiography at Hospital Israelita Albert Einstein, São Paulo, Brazil
*Corresponding author: Claudia Cosentino Gallafrio, Department of Echocardiography at Hospital Israelita Albert Einstein, Av. Albert Einstein, 627/701, Morumbi - CEP 05652 – 900, São Paulo – SP - Brazil
Received: July 22, 2022; Accepted: August 22, 2022; Published: August 29, 2022
Abstract
Background: Despite technological advances in fetal echocardiography, the assessment of the aortic arch is challenging due to the particularities of fetal anatomy and physiology. Both isolated dilatation of right heart chambers, direct visualization and measurements of the aortic arch are related to left obstructive heart disease, such as Coarctation of the Aorta (CoAo). So far, there is still no consensus in the published literature about which echocardiographic finding is of greater value for prenatal diagnosis of these changes.
Objectives: To determine possible correlation between right cardiac chambers dilatation and direct aortic arch measurements with the presence of postnatal CoAo.
Methods: A retrospective study of fetal and postnatal echocardiograms was carried out at Hospital Israelita Albert Einstein (HIAE) between 2011 and 2017. Morphological and functional parameters of the fetal circulation and the transitional circulation period in the newborn were analyzed. Fetal echocardiogram findings were compared with postnatal echocardiograms (presence or absence of congenital heart disease).
Results: Of 2,869 exams performed, 22 fetuses with right chamber dilatation were born at our institution; 20 (90%) had postnatal echocardiogram with normal findings, and only 2 newborns (10%) presented aortic arch obstruction, 1 newborn had critical obstruction and required neonatal intervention. Among all fetal echocardiographic parameters evaluated, only the direct measurement of the aortic isthmus showed correlation with postnatal changes.
Conclusion: In our series, isolated dilation of right heart chambers on fetal echocardiography was not associated with CoAo, but the association of right heart chamber dilatation with aortic arch diameter reduction was, however, more studies with larger numbers of patients are still needed.
Keywords: Coarctation of the aorta; Fetal heart; Prenatal diagnosis
Introduction
In normal fetuses, the size of the cardiac chambers is proportional during most part of the gestation [1-2]. However, with the advancing of gestational age and placental aging, there is an increase in the size of the right heart chambers with greater intensity in the last gestational trimester [3].
Dilatation of the right heart chambers in the first and second trimesters of gestation is an uncommon finding(3)in routine obstetric ultrasonography. In addition, this may indicate the presence of obstructive congenital heart diseases, such as coarctation of the aorta (CoAo) [2,4], which is an indication for fetal echocardiography [5,6].
Currently, echocardiography devices provide images with higher resolution, the fetal diagnosis of some heart diseases remains a challenging [7,8] due to the physiology of the circulation and the imaging characteristics in fetuses [9,10]. This difficulty is particularly more prominent in the evaluation of the aortic arch, which can be aggravated by the presence of the ductus arteriosus (ductal arch) and by technical limitations such as fetal position and echocardiographic window.
Fetal circulation is different from the newborns. In extrauterine life the ventricles function in series with right ventricular output equal to the left, however, in fetal life the circulation is in parallel and it includes four shunts: placenta, ductusvenosus, foramen ovale, and ductus arteriosus. Therefore, the left ventricle is responsible for only 40% of the combined cardiac output, and of this output, 90% is directed to the head and upper limbs. Only 10% of output is directed to the is thmic region, which justifies the smaller diameter of the aorta in this region [11]. This small diameter issue, associated with the presence of the ductal arch that directs the pulmonary trunk flow to the descending aorta, makes the diagnosis of CoAo difficult in this period [12].
Fetal diagnosis of severe CoAo is extremely important, as it is a frequent cause of cardiogenic shock in newborns with closure of the ductus arteriosus. Despite advances in fetal echocardiography, less than 40% of cases are diagnosed prenatally [13-16].
Another aspect that must be considered is the large number of false-positive diagnoses [17,18] in which significant right chamber dilatation is not associated with heart disease in the postnatal period. This aspect is a result of the complex multifactorial interaction of fetus and placental circulation. The false positive results cause great emotional stress to parents and unnecessary use of resources.
In the published literature, studies on the association between disproportion of right/left heart chambers and aortic coarctation are still controversial. They often include a small number of patients and no consensus exist about which parameter is more appropriate to predict the diagnosis and severity of CoAo in the prenatal period [14,19,20].
The aim of this study was to determine a possible correlation between right chambers dilatation and direct aortic arch measurements with the presence of postnatal CoAo.
Methods
This was a retrospective study conducted between 2011 and 2017of the echocardiograms of fetuses with isolated right ventricular dilatation at any gestational age. All examinations were reviewed by a blinded observer to postnatal diagnosis. The study was approved by the Ethics and Research Committee of the institutional where the study was conducted.
All patients who underwent fetal echocardiography with isolated right heart chamber dilation and who were born at Hospital Israelita Albert Einstein (HIAE), Sao Paulo, Brazil, were included. We excluded fetal echocardiograms without postnatal examinations at HIAE, and those associated with other congenital heart diseases besides CoAo, as well as signs of restricted ductus arteriosus or foramen ovale flow and those with fetal arrhythmia.
Prenatal Data
The following data were obtained from questionnaires answered by the mothers before the examination: gestational age, indication for the examination, obstetric history, use of medicines, history of congenital heart disease, possible changes in obstetric ultrasound, and in vitro fertilization were inquired.
Echocardiographic measurements of fetal echocardiography:
Fetal and postnatal echocardiography examinations were performed using Philips machines: iE33 and Epic 7 (Philips ultrasound, Inc; USA).
The echocardiographic images were stored in DICOM (Digital Imaging and Communication in Medicine), in the HIAE database. And reviewed by two echocardiographers qualified by the Department of Cardiovascular Imaging (DIC) of the Brazilian Society of Cardiology, level 3, according to the recommendations of the American Society of Echocardiography, using the Horos Image Viewer system, V.1.1.7. The mean of 3 measurements obtained were used. The following echocardiographic planes were evaluated:
Bidimensional assessment:
Apical 4-chambers:
• Longest longitudinal axis of the right and left ventricles comprised between the mitral/tricuspid valve and the cardiac apex (inlet length).
• Largest diameters of the cavities measured in the trabecular portion of each ventricle (end-diastolic dimension).
• Diameters of the mitral and tricuspid valve annuliat the telediastolic, before their closures (Figure 1).
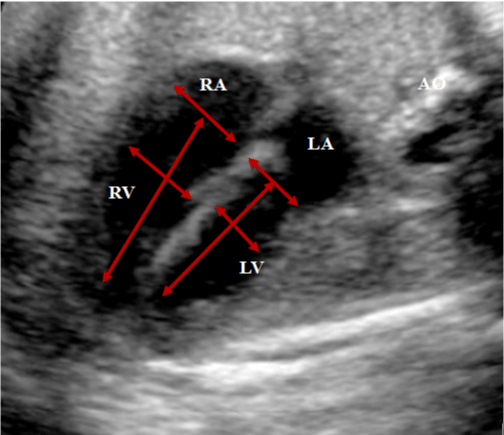
Figure 1: Apical 4-chambers plane, showing the measurements of the
diameters of the rings of the mitral and tricuspid valves in thetelediastolic.
Measurements of the end-dasitolic dimensions of the cavities in the trabecular
portion of each ventricle, and measurements of the largest longitudinal axis of
the right and left ventricles between the mitral/tricuspid valve, and the cardiac
apex (inlet-length).
RA: right atrium; LA: left atrium; AO: aorta; RV: right ventricle; LV: left ventricle
Paraesternal plane:
• Diameters of the aortic and pulmonary valve annuli: measured at systole. (Figure 2 - A and B).
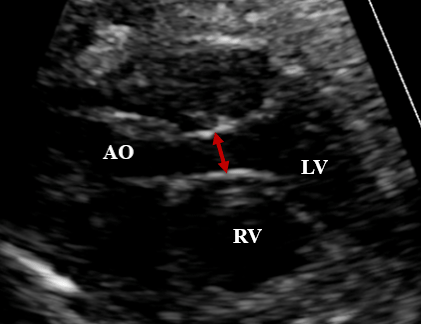
Figure 2: A –Paraesternal longitudinal plane: left ventricle outflow tract
with the measurement of the aortic annulus during systole. B –Paresternal
short-axis plane, showing the right ventricle outflow tract, with the pulmonary
annulus and pulmonary trunk measurements during systole.
AO: aorta; PT: pulmonary trunk; RV: right ventricle; LV: left ventricle.
• Diameter of the pulmonary trunk in systole, in the specific plane of the right ventricle outflow tract (Figure 2 - B).
• Evaluation of the aortic arch, measuring the diameters of the ascending aorta, transverse arch and aortic isthmus in the longitudinal plane. (Figure 3).
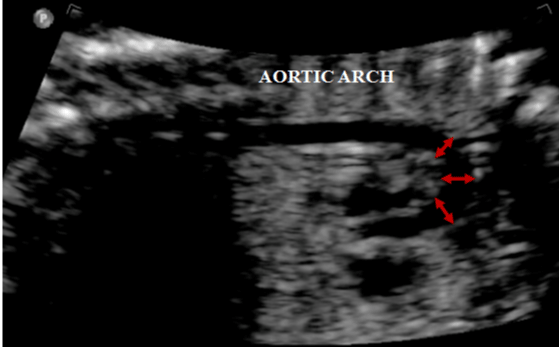
Figure 3: Longitudinal plane of a normal-sized aortic arch, with the
measurements of the ascending aorta, transverse arch, and isthmus.
• Color Doppler evaluation (Figure 4):
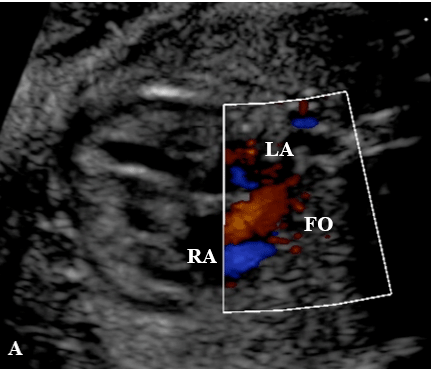
Figure 4: A - Apical four-chamber plane, showing color flow mapping through
the foramen ovale, with flow directed from the right atrium to the left atrium. B
- Three-vessel plane showing the direction of flow in the aorta and pulmonary
trunk at color Doppler.
RA: right atrium; LA: left atrium; AO: aorta; PT: pulmonary trunk
a. Direction of flow through foramen ovale in apical 4-chamber plane.
b. Direction of flow in the aortic arch in the longitudinal plane and three-vessel-trachea view (3VT).
c. Direction of flow in the ductus arteriosus in the longitudinal plane and 3VT.
Postnatal Data
In the neonatal period, postnatal echocardiograms were reviewed for the presence or absence of left obstructive congenital heart disease.
Statistical Analysis
Data were expressed as mean and standard deviations, or median and variations for continuous variables, and percentages for categorical variables. The Mann-Whitney test was adopted for the comparison between the groups with and without postnatal heart disease. Intra- and interobserver variation was calculated using the correlation coefficient in 10 random examinations. A p value of <0.05 was considered significant.
Results
Of the 2,869 fetal echocardiograms performed at HIAE in the period described, 67 of them showed isolated dilation of right heart chambers (2.3%), however; only 22 patients were born in our institution. Most of mothers were primipara (52%) with median age of 35 years (24-48). The median gestational age was 31 weeks (20 - 36 weeks).
The most frequent indication for fetal echocardiography was maternal age (48%)followed by right heart cavity dilatation on obstetric ultrasound (19%), twin pregnancy (9.5%), family history of congenital heart disease (9.5%), and other maternal causes (14%). None of the examinations were indicated because of intrauterine growth restriction or in-vitro fertilization. According to postnatal echocardiographic findings patients were separated into two groups. Group 1 - patients without heart disease with structurally normal heart. Group 2 - patients with aortic arch obstruction.
Of the 22 fetuses included in the study, 20 (90%) were in group 1 and only 2 (10%) composed the group 2, and only 1 fetus had critical obstruction and required neonatal intervention.
In group 1, the mean diameter of the tricuspid valve annulus was 11.56 mm (±3.17) of the mitral valve annulus was 8.59 mm (±1.54) and mean tricuspid/mitral ratio of 1.36 (±0.32).The mean of Right Ventricle (RV) end-diastolic dimension was 12.4 mm (±2.92) and the Left Ventricular (LV) was 8.09 mm (1.64), with a mean RV/LV enddiastolic dimension ratio of 1.51 (±0.29). The mean RV inlet length was 19.84 mm (±3.35) and LV, 19.00 mm (±4.59) with a RV/LV inlet length ratio of 1.07 (±0.15). The mean diameter of the Pulmonary valve Annulus (PA) was 7.10 mm (±1.76) and the mean diameter of the aortic valve annulus (Ao) was 4.99 mm (±0.87), with a PA/Ao ratio of 1.42 (±0.24). The mean diameter of the Pulmonary Trunk (PT) was 7.90 mm (±1.87) and of the ascending aorta (AscAo) was 5.44 mm (±1.24), with a PT/ AscAo ratio of 1.44 (±0.32). The mean diameter of the transverse arch was 4.83 mm (±0.86), and the longitudinal isthmus was 3.91 mm (±0.81) (Table 1).
FINDINGS
GROUP 1 (n=20)
GROUP 2 (n=2)
P
Gestational age (weeks)
30.7 ± 4.15
29 ±5.66
0.701
Main entrance measures (mm)
Mean tricuspid valve (TV)
11.56 ± 3.17
11.73 ± 6.03
0.866
Mean mitral valve (MV)
8.59 ± 1.54
8.50 ± 0.90
0.952
TV/MV relationship
1.36 ± 0.32
1.35 ± 0.57
0.779
Ventricle dimensions(mm)
Mean RV end-diastolic dimension
12.14 ± 2.92
10.12 ± 4.50
0.485
Mean LV end-diastolic dimension
8.09 ±1.64
7.72 ± 1.96
0.701
Mean RV/LV end-diastolic dimension ratio
1.51 ± 0.29
1.28 ± 0.26
0.260
Mean RV inlet-length
19.84 ± 3.35
20.52 ± 7.99
0.952
Mean LV inlry-length
19.00 ± 4.59
17.20 ± 4.57
0.701
Mean RV/LV inlet-length ratio
1.07 ± 0.15
1.17 ± 0.15
0.424
Outflow tracts evaluation (mm)
Mean Pulmonary valve (VP)
7.10 ± 1.76
7.05 ± 1.63
0.952
Mean Aortic valve (VA)
4.99 ± 0.87
3.90 ± 1.65
0.424
Mean VP/VA relationship
1.42 ± 0.24
1.89 ± 0.38
0.104
Mean Pulmonary trunk(PT)
7.90 ± 1.87
7.07± 2.12
0.554
Mean Ascending aorta mean (AscAo)
5.44 ± 1.24
4.42 ± 2.24
0.701
Mean PT/Asc AOratio
1.44 ± 0.32
1.70 ± 0.38
0.485
Aortic arch measures (mm)
Mean Longitudinal isthmus
3.91 ± 0.81
2.38 ±0.78
0.057
Mean transverse arch mean (TA)
4.83 ± 0.86
3.92 ± 0.87
0.238
Mean PT/TS relationship
1.56 ± 0.49
1.79 ± 0.14
0.686
Asc: ascending aorta; TA: transverse aortic arch; PT: pulmonary trunk; VA: aortic valve; RV: right ventricle; LV: left ventricle; MV: mitral valve; PV: pulmonary valve; VT: tricuspid valve.
Data correspond to the mean, with the standard deviation, as well as the - p value.
Table 1: Fetal echocardiography data comparing the postnatal findings of structurally normal hearts (Group - 1) and hearts with coarctation of the aorta (Group - 2).
Interobservers evaluation
Intraobservers evaluation
Mean Tricuspid Valve (VT)
CCI 0.99, 95% IC 0.95-0.99
CCI 0.99, 95% IC 0.97-0.99
CCI 0.88, 95% IC 0.51-0.97
CCI 0.96, 95% IC 0.83-0.99
Mean Mitral valve (MV)
CCI 0.97, 95% IC 0.91-0.99
CCI 0.99, 95% IC 0.98-0.99
CCI 0.93, 95% IC 0.75-0.98
CCI 0.98, 95% IC 0.93-0.99
Mean RV end-diastolic dimension
CCI 0.96, 95% IC 0.86-0.99
CCI 0.97, 95% IC 0.90-0.99
CCI 0.95, 95% IC 0.82-0.98
CCI 0.97, 95% IC 0.90-0.99
Mean LV end-diastolic dimension
CCI 0.98, 95% IC 0.95-0.99
CCI 0.99, 95% IC 0.96-0.99
CCI 0.83, 95% IC 0.33-0.96
CCI 0.90, 95% IC 0.60-0.97
Mean RV inlet-length
CCI 0.98, 95% IC 0.94-0.99
CCI 0.99, 95% IC 0.97-0.99
CCI 0.96, 95% IC 0.84-0.99
CCI 0.98, 95% IC 0.90-0.99
Mean LV inlet-length
CCI 0.94, 95% IC 0.78-0.98
CCI 0.93, 95% IC 0.72-0.98
CCI 0.94, 95% IC 0.88-0.99
CCI 0.94, 95% IC 0.88-0.99
Asc: ascending aorta; TA: transverse aortic arch; ICC: inter- and intraclass correlation coefficient; CI: confidence interval; PT: pulmonary trunk; VA: aortic valve; RV: right ventricle; LV: left ventricle; MV: mitral valve; PV: pulmonary valve; VT: tricuspid valve.
Table 2: Inter- and intraobserver correlation of echocardiographic measurements.
In group 2, the mean diameter of the tricuspid valve annulus was 11.73 mm (±6.03), the mitral valve annulus was 8.50 mm (±0.9), with a tricuspid/mitral ratio of 1.35 (±0.57). The mean RV end-diastolic dimension was 10.12 mm (±4.5) and the LV was 7.72 mm (±1.96), with a mean RV/LV end-diastolic dimension ratio of 1.28 (±0.26). The mean RV inlet length was 20.52 mm (±7.79) and LV, 17.20 mm (±4.57) with a RV/LV inlet length ratio of 1.17 (±0.15). The mean diameter of the Pulmonary valve Annulus (PA) was 7.05 mm (±1.63), and the mean diameter of the aortic valve annulus (Ao) was 3.90 mm (±1.95), with an AP/Ao ratio of 1.89 (±0.38). The mean diameter of the Pulmonary Trunk (PT) was 7.07 mm (±2.12) and of the ascending aorta (AscAo) was 4.42 mm (±2.24), with a PT/ AscAoratio of 1.70 (±0.38). The mean diameter of the transverse arch was 3.92 mm (±0.87) and of the longitudinal is thmus was 2.38 mm (±0.38).
When prenatal data were compared with the presence or absence of heart disease, only the measurements of the longitudinal aortic isthmus were statistically significant (3.91 mm +- 0.81 vs. 2.38 mm +- 0.78; p = 0.057) (Table 1).
Discussion
Fetal diagnosis of CoAo is challenging especially for non-critical cases. Both disproportion of right and left heart diameters and direct measurements of the aortic arch, including the longitudinal isthmus, are markers of possible cardiac malformations.
In our series, only the reduction in diameter of the longitudinal isthmus (Figure 5) in fetal echocardiography showed a statistically significant correlation with CoAo after birth. This finding is in agreement with a study published by Hornberger et al (4) that retrospectively evaluated 124 fetuses and detected five of them with CoAo. In this series, only the diameters of the transverse arch and the isthmic region showed statistically significant changes. The percentile was below 3 for gestational age. Another finding was the result of the division of the left carotid diameter by the transverse arch which also showed statistical significance [4].
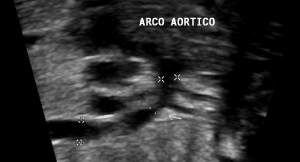
Figure 5: Longitudinal plane of the aortic arch, showing narrowing of the
isthmic region (arrow).
It is important to emphasize that the good quality of the fetal aortic arch image with adequate demonstration of isthmic narrowing seems to be the most determinant point in the correct diagnosis of CoAo. This was demonstrated in another retrospective study [18] that evaluated 615 fetuses and reported 54 fetuses with CoAo. In this study, the relations of the pulmonary artery to aorta, right ventricle to left ventricle, and mitral to tricuspid diameters were not statistically significant and they were also not useful to distinguish false positive cases [18].
However, the right predominance in fetal echocardiography has been related to the presence of postnatal CoAo mainly in examinations performed in the second gestational trimester; excluding the physiological increase found in the last trimester that may be secondary to the complex relationship between maturity, and placental insufficiency with the fetal circulation dynamics. According to Jung et al [1] among fetuses who had right ventricular dilatation in the second trimester of gestation, 60% of them had CoAo in the postnatal period. However, the majority of fetuses with right chamber enlargement in the last trimester had normal postnatal echocardiograms (86.2%). A significant part of our sample that was in the last gestational trimester may have limited our analysis.
We identified that the association between disproportions of heart chambers with aortic arch narrowing seemed to increase the accuracy of fetal diagnosis of CoAo. In another retrospective study, Quaterman et al (14) evaluated 35 fetuses that were divided into two groups, one group with surgical intervention in the neonatal period and the other without it The authors suggested cutoff values to predict critical aortic arch obstruction in fetuses with right ventricular dilatation, particularly due to the relationship between LV/RV diameters that was less than or equal to 0.6, and associated with transverse arch and a diameter less than 3 mm for pregnancies at 30 weeks or greater. Thus, they determined which fetuses should be referred to tertiary centers with cardiologists for initiation of prostaglandin after birth. Such finding was confirmed in a systematic review published in 2017 [21], where 12 studies were included and evaluated 922 fetuses with suspected CoAo. Of these, 283 had the diagnosis confirmed on postnatal examination. The presence of aortic arch hypoplasia in fetuses with disproportion of right/left heart diameters proved to be one of the best parameters for detecting CoAo, with a sensitivity of 90% and specificity of 87.1% [21]. The narrowing of the isthmic region presented high specificity, 97.7%, however, with low sensitivity [21].
Conclusion
The isolated disproportion between right and left heart diameters did not correlate with postnatal congenital heart disease. However, its association with the measurement of the isthmus in the plane of the longitudinal aortic arch seems to be more efficient in the diagnosis of fetal CoAo. Prospective studies with larger numbers of patients are warranted to determine if any single fetal echocardiographic parameter can more accurately diagnose prenatal CoAo, and, consequently, avoid the waste of resources and unnecessary emotional stress to families.
Limitations of the Study
Our study was retrospective and presented few patients who met the inclusion criteria. In addition, many of our patients with abnormal fetal scans were born in other institutions and they were excluded from the study sample. Other limitations were the advanced gestational age in a significant number of patients, which may have limited the statistical significance of disproportion of the heart chambers in isolation, and the lack of imaging with measurement of the aortic isthmus in the 3-vessel plane with trachea in all examinations.
References
- Jung E, Won H, Lee PR, Kim A, Park I. Clinical implication of isolated right dominant heart in the fetus. Prenatal Diagnosis. 2007; 27: 695-698.
- Hornung TS, Heads A, Hunter AS. Right Ventricular Dilatation in the Fetus: A Study of Associated Features and Outcome. Pediatric Cardiology. 2001; 22: 215-217.
- Sivanandam S, Nyholm J, Wey A, Bass JL. Right Ventricular Enlargement In Utero: Is It Coarctation?. Pediatric Cardiology. 2015; 36: 1376-1381.
- Hornberger LK, Sahn DJ, Kleinman CS, Copel J, Silverman NH. Antenatal diagnosis of coarctation of the aorta: a multicenter experience. Journal of the American College of Cardiology. 1994; 23: 417-423.
- Molina FS, Nicolaides KH, Carvalho JS. Two- and three-dimensional imaging of coarctation shelf in the human fetus. Heart. 2008; 94: 584.
- Head CEG, Jowett VC, Sharland GK, Simpson JM. Timing of presentation and postnatal outcome of infants suspected of having coarctation of the aorta during fetal life. Heart. 2005; 91: 1070-1074.
- Bronshtein M, Zimmer EZ. Sonographic diagnosis of fetal coarctation of the aorta at 14–16 weeks of gestation. Ultrasound in Obstetrics and Gynecology. 1998; 11: 254-257.
- Gómez-Montes E, Herraiz I, Mendoza A, Escribano D, Galindo A. Prediction of coarctation of the aorta in the second half of pregnancy. Ultrasound in Obstetrics & Gynecology. 2013; 41: 298-305.
- Franklin O, Burch M, Manning N, Sleeman K, Gould S, Archer N. Prenatal diagnosis of coarctation of the aorta improves survival and reduces morbidity. Heart. 2002; 87: 67-69.
- Matsui H, Mellander M, Roughton M, Jicinska H, Gardiner HM. Morphological and Physiological Predictors of Fetal Aortic Coarctation. Circulation. 2008; 118: 1793-1801.
- Rosenthal E. Coarctation of the aorta from fetus to adult: curable condition or life long disease process?. Heart. 2005; 91: 1495-1502.
- Hornberger LK, Weintraub RG, Pesonen E, Murillo-Olivas A, Simpson IA, Sahn C, et al. Echocardiographic Study of the Morphology and Growth of the Aortic Arch in the Human Fetus: Observations Related to the Prenatal Diagnosis of Coarctation. Circulation. 1992; 86: 741-747.
- Sato M, Tsukimori K, Fujita Y, Morihana E, Fusazaki N, Takahata Y, et al. Prenatal Diagnosis of Coarctation of the Aorta Using 4-Dimensional Fetal Echocardiography With Power Doppler Imaging and Spatiotemporal Image Correlation. Journal of Ultrasound in Medicine. 2013; 32: 719-721.
- Quartermain MD, Cohen MS, Dominguez TE, Tian Z, Donaghue DD, Rychik J. Left ventricle to right ventricle size discrepancy in the fetus: the presence of critical congenital heart disease can be reliably predicted. Journal of the American Society of Echocardiography: official publication of the American Society of Echocardiography. 2009; 22: 1296-1301.
- Gardiner H, Chaoui R. The fetal three-vessel and tracheal view revisited. Seminars in fetal & neonatal medicine. 2013; 18: 261-268.
- Buyens A, Gyselaers W, Coumans A, Nasiry SA, Willekes C, Boshoff D, et al. Difficult prenatal diagnosis: fetal coarctation. Facts, Views & Vision in ObGyn. 2012; 4: 230 - 236.
- Paladini D, Volpe P, Russo MG, Vassallo M, Sclavo G, Gentile M. Aortic coarctation: prognostic indicators of survival in the fetus. Heart. 2004; 90: 1348-1349.
- Sharland GK, Chan KY, Allan LD. Coarctation of the aorta: difficulties in prenatal diagnosis. British Heart Journal. 1994; 71: 70-75.
- Durand I, Deverriere G, Thill C, Lety AS, Parrod C, David N, et al. Prenatal Detection of Coarctation of the Aorta in a Non-selected Population: A Prospective Analysis of 10 Years of Experience. Pediatric Cardiology. 2015; 36: 1248-1254.
- Slodki M, Rychik J, Moszura T, Janiak K, Respondek-Liberska M. Measurement of the Great Vessels in the Mediastinum Could Help Distinguish True From False-Positive Coarctation of the Aorta in the Third Trimester. Journal of Ultrasound in Medicine. 2009; 28: 1313-1317.
- Familiari A, Morlando M, Khalil A, Sonesson SE, Scala C, Rizzo G, et al. Risk Factors for Coarctation of the Aorta on Prenatal Ultrasound: A Systematic Review and Meta-Analysis. Circulation. 2017; 135: 772–85.