
Review Article
Austin J Clin Immunol. 2016; 3(1):1027.
Vaccine for Human Papillomavirus
Getinet Melkamu*, Gelaw Baye and Assefa Abate
Department of Medical Microbiology, University of Gondar, Ethiopia
*Corresponding author: Getinet Melkamu, Department of Medical Microbiology, School of Biomedical and Laboratory Sciences, College of Medicine and Health Sciences, University of Gondar, Gondar, Ethiopia
Received: January 08, 2016; Accepted: January 30, 2016; Published: February 02, 2016
Abstract
Human Papillomavirus (HPV) is among the large group of epitheliotropic viruses with more than 100 different types. There are about 40 HPV types infecting the human body with the association of oncogenic risk. The development of cervical cancer is contributed by a variety of factors even though HPV is the primary etiologic agent. Cervical cancer is the second most common cancer among women in the developing countries and estimated to affect approximately 500 000 women worldwide each year, of whom 80% live in developing countries. All cervical cancer cases are resulted from genital infection of HPV and shared similar risk factors with Human Immunodeficiency Virus (HIV).
There is no effective treatment for HPV infections and immunization against HPV may be paramount important to prevent this infection. The main aim of this review is to compile data on HPV vaccinations. Vaccination of target groups is indispensable aspect of HPV prevention. The two currently available HPV vaccines, Gardasil and Cervarix are designed to protect against four types of HPV. Each of them produce Virus Like Particles (VLPs) prepared from recombinant vaccine technology. These vaccines are most effective during childhood or adolescence, but adults also can be benefited. The HPV vaccine is highly immunogenic have the ability to circumvent the viral immune evading mechanisms. The vaccine has no serious side effects, targeted for reproductive age groups, highly efficacious in preventing new infections of most common oncogenic HPV types and will dramatically reduce the rates of HPV associated cancer. The vaccine is widely and properly delivered to the target population particularly for developing countries. So feature researches should be focus on the introduction of HPV vaccines in to routine immunization.
Keywords: Human papillomavirus; HPV vaccine
Introduction
The papillomaviruses are members of the family Papovaviridae, non enveloped and double strand Deoxy ribonucleic Acid (DNA) virus [1]. Papillomaviruses can infect humans as well as animals. Papillomaviruses that infect humans are known as human papillomavirus. The HPV usually causes a variety of benign papillomatous lesions of the skin, squamous mucosa and induces tumors in their natural host [2].
Human papillomavirus is among the large group of epitheliotropic viruses of more than 100 different types, 40 are known to cause infections to humans. Some of them are associated with oncogenic risk [3]. Based on their oncogenic potential genital HPV can be classified in to Low-Risk (LR) and High-Risk (HR) genotypes. The HPV 16 and 18 are among the HR types and responsible for the majority of HPV associated cancers of the ano-genital tract, while HPV 6 and 11 are the LR types which cause a substantial proportion of low-grade cervical dysplasia. More than 90% of genital warts are also due to LR types. On the other hand 99% of cervical cancer is due to HR types [4].
Though, HPV is the primary etiologic agents of cervical cancer, variety of factors contribute for the development of cervical cancer. Several studies revealed that HIV infection in women is associated with an increased risk of HPV associated malignancies and cervical cancer [5]. Different findings also strongly support a dose response relationship between host immune status and the risk of HPV-related tumorigenesis [6]. The HIV-positive women have a higher prevalence of cervical epithelial cell abnormality and high progression rate of precancerous cervical lesions [7]. Cervical cancer is the aggravation factor for the progression of HIV and vice versa [8]. HIV treatment guidelines recommend annual Pap tests for HIV infected women [9].
Women who acquire HPV infection do not develop invasive cancer. Most infections are transient and approximately 90% of infections clear within 2 years [10]. The peak prevalence of HPV infection occurs in the late teens and early twenties following onset of sexual activity [11], that can be easily spread through direct skinto- skin contact during vaginal, anal and oral sex [12]. In fact, more than half of sexually active people are infected with one or more HPV types at some point in their lives [13]. The HPV infection is mainly squamous epithelial cell surfaces of skin or mucosa, such as the interior mouth, throat, tongue, tonsils, vagina, cervix, vulva, penis, and anus. Infection is initiated when infectious particles binds with basal layer of the epithelium. Since, HPV infection is more of asymptomatic and its persistence nature, infected people do not know they have it [14,15].
The presence of HPV infection can be detected by using screening methods based on epithelial abnormality or DNA testing technologies [16]. Infection with HPV leads in to other complications like infertility [17]. Cervical cancer is the second most common cancer among women in the developing countries and estimated to affect approximately 500 000 women worldwide each year, of whom around 80% of cases occur in developing countries. Moreover, all cervical cancer cases are as a result of genital HPV infection [18].
Understanding the existence and transmission of HPV are indispensable aspects of its prevention. Poor comprehensive knowledge about cervical cancer, associated risk factors and cost incurred for cervical cancer cases were assessed in Gondar and Tikur Anbessa hospital respectively. The result revealed that poor knowledge and high cost incurred per patient (cost per patient was $407.20) were identified [19,20].
Infections associated with HPV are not treated; treatment option is directed on the lesions of genital warts, cervical, vaginal, and vulvar cancer precursors are by applying local approaches like:- cryotherapy, electrocautery, laser therapy, and surgical excision. These treatments can reduce the infectiousness but not eliminate HPV [21]. The HPV infections also largely shielded from the host immune response because they are restricted to the epithelium. The best characterized and most type-specific antibodies are those directed against conformational epitopes of the L1 capsid protein assembled as VLPs [22].
Immunization against HPV may have greatest value in developing countries, where 80% of the global burden of cervical cancer occurs each year and where Pap screening programs are not practice. Two types of prophylactic vaccine are currently being developed [23]. Vaccine safety and efficacy are some of the advantages. On the other hand the vaccine has some disadvantages like: it doesn’t prevent all strains, little is known about long term effects and it has few side effects [24]. The aim of this review was to summarize various aspects of immunity against HPV and the use of vaccine.
Immune response against HPV
Transient genital HPV infections are relatively common in young sexually active women but most of these infections are subclinical and spontaneously resolve [25]. Of the HPV-infected women who develop persistent infections, only a few will progress to ICC. Natural immune response is adequate to clear disseminated HPV infection. Although the natural immunity is responsible to clear the infection, the mechanisms of actions are largely undefined. Different studies implicate the involvement of both innate and adaptive immunity. The human female reproductive tract has the capacity to mount both humoral immunity and Cell Mediated Immunity (CMI) following infection with HPV [26].
The role of innate immune system during early stages of HPV infections creates a pro-inflammatory microenvironment. This is due to the recruiting of innate immune cells to eliminate the infected cells and initiating an effective acquired immune response [27]. Most benign lesions mount an effective CMI and the lesions regress by a CD4+ T cell dominated T helper (Th) 1 response. The central role of the CD4+ T cells can be demonstrated, when an increased prevalence of HPV infections in immunocompromised individuals [26]. Keratinocytes the cells infected by HR HPV are equipped with different Pattern Recognition Receptors (PRRs). This allows to recognize invading pathogens and to activate the immune system [27]. Serum neutralizing antibody to the major capsid protein L1 usually develops after the induction of successful CMI. These antibody and cell mediated responses are protective against subsequent viral challenge in natural infections [26].
However, HPV has a wide range of strategies for evading immune response. It can generate anti-inflammatory microenvironment to down-regulate adhesion molecules and chemo-attractants [27]. The persistent nature of HPV infection is also an effective evasion mechanism of innate immunity [28]. More over HPV develop induced expression of the cellular protein like Ubiquitin Carboxyl- Terminal Hydrolase L1 (UCHL1) in keratinocytes to suppresses the activation of signals and downstream of the pathogen receptor. As a result transcription factor activation and gene expression of HPV are reduced, in particular pro-inflammatory cytokine and lowers the attraction of immune cells and thereby the chance of HPV-infected cells to be recognized and eliminated and as such enables HPV to persist [26].
Several studies shown that HR HPV infection also down regulate IFN-α inducible gene expression. The HPV 16 E6 and E7 oncoproteins directly interact with components of the interferon signaling pathways, even in the absence of viral-induced cytolysis and cell death. The type 1IFNs (INF-α and β) is powerful, generic and innate antiviral defense system. The antiviral effects of IFNs have anti proliferative and immuno stimulatory properties that act as a bridge between innate and adaptive immunity [29]. Human papillomavirus is also able to inhibit the secretion of Interferon gamma (IFN-γ) and the expression of some innate immune cell receptors like NKs. Immunoglobulin-Like Transcript 2 (ILT2) is regulatory receptors that participate in the pathogenesis of viral infections [30]. T-cell responses to E2, E6 and E7 are lost or reduced in HSIL and invasive carcinoma. Even if HPV antigen-specific cytotoxic T-cells are released, the regulatory T-cells increasingly dominate the lesions and reduce the killer defense response. The challenge for therapeutic vaccines for HPV associated disease is to reverse this immunologically suppressive microenvironment and allow the cytotoxic killers to access the infected cells [28]. The overall innate immune response is summarized in Figure 1.
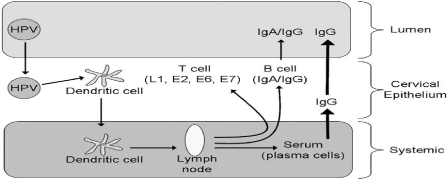
Figure 1: Simplified summary of innate immunological response to HPV
infection [30].
The administration of adjuvant, such as Toll-Like Receptors (TLR) agonists and alpha-galactosylceramide reverse the antiinflammatory microenvironment and promote immune response. Prophylactic vaccines consisting of HPV L1 VLPs generate both CMI and humoral immunity. This immunity has greater than 95 % efficacy against HPV associated disease and therefore circumvent the immune evasion strategies of the virus [28]. Similarly the regulatory function of ILT2 on cell proliferation and IFN-γ production increase in the expression of ILT2 by NK and CD3+ CD56+ lymphocytes and monocytes after quadrivalent HPV vaccine immunization and also increased the proportion of CD3- CD56+ ILT2+ NKs [30].
The HPV vaccines
The two currently available HPV vaccines, Gardasil and Cervarix, produce VLPs and prepared from recombinant vaccine technology in yeast and Baculo virus from empty protein shells. These vaccines are not live since it doesn’t contain any live biological product or DNA making infectious. It is a prophylactic vaccine that is designed to prevent initial HPV infection [39,40]. The first HPV vaccine Gardasil (quadrivalent or HPV4) was approved by United States Food and Drug Administration (US FDA) in 2006. On the same year it was licensed in several countries and later followed by Cervarix (bivalent or HPV2) in 2009. The success of vaccination programs can be attributed to both direct protection for individuals who are immunized and to indirect protection or so-called ‘herd immunity’ [31,32].
Human papillomavirus vaccination was introduced into routine immunization schedule in the United States in the late 2006 for females from 11 to 12 ages and a catch-up vaccination for those from 13 to 26 ages. Together with regular gynecological screening, the treatment of precancerous lesions has been very effective in preventing ICC. Center for Disease prevention and Control (CDC) recommends companies like GlaxoSmithKline and Merck for Cervarix and Gardasil respectively and FDA is responsible for licensing [33,34].
These two well known vaccines can efficiently prevent infection with HPV. Gardasil is designed to protect against HPV types 6, 11, 16 &18 which cause 70% of cervical cancer in females and 90% of genital warts both males and female. Cervarix provides protection against HPV16 & 18 types. Gardasil has an advantage over Cervarix by covering 6 and 11 HPV types and licensed for males on the other hand Cervarix has a cross protection profile [34].
Currently FDA approved an expanded age indication for Gardasil9 use in males 16 through 26 years of age, for the prevention of anal cancer caused by HPV types 16, 18, 31, 33, 45, 52 and 58, precancerous or dysplastic lesions caused by HPV types 6, 11, 16, 18, 31, 33, 45, 52 and 58, and genital warts caused by HPV types 6 and 11. GARDASIL 9 is already approved for use in boys 9 through 15 years of age for the prevention of these diseases. GARDASIL 9 is also approved for use in girls and young women 9 through 26 years of age for the prevention of cervical, vulvar, vaginal, and anal cancers caused by HPV 16, 18, 31, 33, 45, 52 and 58, precancerous or dysplastic lesions caused by HPV 6, 11, 16, 18, 31, 33, 45, 52, and 58, and genital warts caused by HPV types 6 and 11 [35].
Besides commercial preventive HPV vaccines, Gardasil and Cervarix, therapeutic HPV vaccines can eliminate preexisting lesions and infections by generating cellular immunity against HPV-infected cells. Human papillomavirus E6 and E7 oncoproteins represent ideal targets for therapeutic intervention because of their constitutive expression in HPV-associated tumors and their crucial role in the induction and maintenance of HPV-associated disease. At this regard there is progress of various therapeutic HPV vaccine approaches, including live vector-based, peptide/protein-based, nucleic acid-based and cell-based vaccines targeting E6 and/or E7 antigens, and their future prospects for the control of HPV-associated malignancies [36]. Viral vectors are attractive vaccines for therapeutic HPV vaccination due to their high immunogenicity. There are many preclinical studies on the efficacy of live viral vectors, such as adenoviruses, alpha viruses, vaccinia viruses vesicular and stomatitis viruses [37].
Target group
Human papillomavirus vaccination is most effective during childhood or adolescence before sexual debut, adults can also get benefit from the vaccine. Both vaccines are extremely effective at preventing infection by the HPV types they cover [38]. Getting the HPV vaccine reduces a women risk of cervical cancer and precancerous growths substantially. Even though men cannot develop cervical cancer, the vaccines have a direct benefit against genital warts and anal cancer. Vaccination of men has an indirect protection of women by reducing HPV transmission. The HPV vaccine does not treat or cure HPV infections who are already infected by one of these HPV types [39].
According to CDC recommendations all women aged 26 years and younger receive three doses of the HPV vaccine (Cervarix or Gardasil). But men age 21 years and younger receive three doses of Gardasil only. The recommendation is for those men who have sex with men or who have a compromised immune system including HIV to receive the HPV vaccine through age 26 if not received earlier. The recommended three doses of HPV vaccine should be given as first dose: between ages 11 and 26, second dose: one to two months after the first dose and third dose: six months after the first dose. Some adults may have received doses of the HPV vaccine in childhood or adolescence. All three doses should be administered to get maximum benefit from HPV infection. Adulthood can be revaccinated if the vaccination schedule was not completed [40].
National Advisory Committee on Immunization (NACI) recommends the primary age groups for vaccination are females aged 9 to 13. Since HPV is well known STI, it is ideal females should be vaccinated before their life time first sexual contact so as to assure the maximum benefit. The NACI also recommend females those exposed for sexual contact could have been took the vaccine and will be benefited until the age of 26 years. The vaccine has still an importance for women who have Pap abnormalities, cervical cancer, ano-genital warts or a known HPV infection. Gardasil may be administered to females over 26 years of age. Males between the ages of 14 and 26 years would also benefit from Gardasil even if they are already sexually active as they may not yet have HPV infection and are very unlikely to have been infected with all four HPV types in the vaccine [41]. The pivotal role in the prevention of cervical cancer includes vaccination of newborns and infants that will be well established with successful working infrastructure [42].
Human papillomavirus vaccines mechanisms of action
Human papillomavirus vaccines contain the major capsid L1 recombinant viral proteins, which self-assemble into VLP resembling HPV. It is mediated by the development of cell mediated and humoral immune responses [43]. Neutralizing antibodies (NAbs) are a result of humoral immunity can sufficient and necessary for protection against viral infections. The overall effects of NABs are shown below (Figure 2).
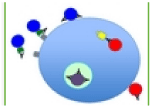
Figure 2: The mechanism of neutralization.
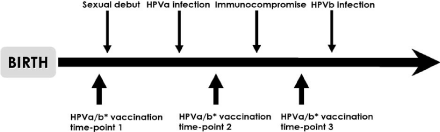
Figure 3: HPV vaccination and timing of development of immunocompromised
state [59].
Neutralization of enveloped viruses blocks viral attachment and entry. Entry can be blocked at different stages. The three blue virions to the left represent enveloped virus particles. The first has an IgG bound to its receptor-binding protein (green). The bound NABs block the docking onto the receptor (grey) on the cell surface. The second virion has already established contact between its receptorbinding protein and the cell-surface receptor. The NAb binds to an epitope on the envelope glycoprotein that may have become exposed after the receptor binding and blocks subsequent steps; these could be interactions with a second receptor or the fusogenic refolding of the envelope glycoprotein. The third blue virion is about to fuse with the cell membrane, but NAbs bound to membrane proximal epitopes on fusogenic proteins (not shown) prevent the completion of this process. The latter two interferences with entry could also occur in endosomes, but hardly the first, unless there are alternative attachment proteins the virus can bind to and thereby get internalized. The purple virion in the endosome is prevented by NAbs from fusing its envelope with the vesicular membrane. Alternatively, this purple virion could represent a naked virus particle, the penetration of which is prevented by the NAbs. The block of infection in the endosome could properly be called a post internalization block of entry; for clarity, entry should refer to transfer of the viral core or capsid, or possibly only genome, into the cytoplasm. The red virion on the cell surface depicts a naked virion that binds to a cell surface receptor and injects the genome into the cytoplasm. This process may occur in vesicles or semi sealed invaginations of the cell surface. If the NAbs have not prevented receptor interactions, they may interfere with the extrusion of the genome. The red virion in the cytoplasm has penetrated an endosomal membrane in complex with the NAb, allowing binding to TRIM21 (yellow box with arrow), which mediates the ubiquitination of the complex, targeting it for proteasomal degradation. Adopted from P.J. Klas Advances in Biology, Volume 2014 (2014), Article ID 157895.
Vaccines are generally less effective in immuno compromised individuals, including pregnant women, the elderly, the malnourished, and those with immune systems damaged by infection (e.g., HIV, measles, and malaria) or treatment (e.g., with corticosteroids or chemotherapy), although substantial immunity can be produced despite these problems, as demonstrated for many of the vaccines from World Health Organization (WHO) Expanded Vaccine Initiative [44].
Gardasil is a sterile preparation for intramuscular injection and contains purified inactive proteins from four HPV types (HPV 6, 11, 16, and 18). These proteins are in structurally indistinguishable to the HPV and can activate the immune system without replicating the virus. Viral proteins can be combined with a catalyst amorphous aluminum hydroxyphosphate sulfate and then 0.5 ml is injected intramuscularly as three separate doses. The vaccine can be administered concomitantly at different sites with different vaccines like Hepatitis B Virus, Diphtheria and Tetanus vaccines, Hormonal contraceptives do not interact but the use of immunosuppressive drugs reduces its efficacy [45].
On the other hand Cervarix is combined with Aluminum hydroxide 3-O-deacyl-4’ monophosphoryl lipid A (AS04) as catalysts. Antigens and AS04 must be injected at the same intramuscular site together or within 24 hr of each other. The catalyst induces local cytokine production leading to increased recruitment of dendritic cells and monocytes and raised numbers of antigen presenting cells in the draining lymph node. The localized and transient nature of the innate immune response supports the acceptable safety profile observed in different studies. Cross-protection against oncogenic HPV types 31/33/45 not contained in the vaccine is also observed [46].
Eligibility and Safety Issues
People should not be vaccinated if they are sensitive to sever allergy for any of the vaccine component. For example yeast for HPV4, having a moderate or severe acute infectious illness, bleeding disorder that causes to bruise or bleed easily or if on medication that thins the blood (anticoagulant therapy), unless otherwise advised by the doctor [47].
The vaccine may have the following side effects local reactions at the site of injection like: - pain, swelling, itching and redness may account 20-90% of cases; fever up to 1000F or above may occur 10- 13% of cases are. Fainting immediately after HPV vaccination has also been noted in some countries and other mild side-effects such as: a head ache, dizziness, diarrhea, pains in muscles also considered and there is no serious adverse effects identified [48].
The impacts of HPV vaccines on HIV patients
Studies have shown that HPV vaccination is safe and immunogenic and does not cause problems for HIV-infected women who got the vaccine. The vaccine is not contraindicated in HIV-infected women [49]. Human immunodeficiency virus and genital HPV infection share similar risk factors. It seems that the presence of HPV infection actually increases the risk for HIV acquisition and transmission and the biological plausibility of this phenomenon is explored in recent publications. There are an increasing number of reports supporting a possible relationship between HPV infection and the risk for HIV acquisition [50,51].
There are also other studies that HIV-infected women have higher prevalence, incidence, viral load of , longer persistence, higher likelihood of multiple subtypes and greater prevalence of oncogenic subtypes of HPV than HIV uninfected women. Meta-analysis in 2008 delineated the rate of cervical HPV infection in HIV infected women with normal cervical cytology varied from more than 55% in South and Central America and Africa to over 30% in Asia, North America, and Europe [52].
HPV vaccination is likely to be more effective if initiated at a time when the patient is less immunosuppressed, either prior to an advanced stage of HIV/AIDS or after initiation of a successful Highly Activated Antiretroviral Treatment (HAART) regimen and even individual may be benefit after their immunity is compromised for unexposed HPV types. For example vaccination against HPV types a and b with time is illustrated in Figure 3 below [53].
HPV vaccination against HPV types a and b (e.g., HPV can be either HPV-16 or -18) at time-point 1, before sexual debut, would potentially benefit all individuals, whether or not they become immunocompromised. HPV vaccination against HPV types a and b at time-point 2, after sexual debut but before onset of immunocompromised among at-risk individuals. May benefit some individuals, particularly if they have not yet been exposed to both HPV-a and HPV-b. Vaccination at time-point 3, after onset of immunocompromised, may be the least effective approach if the degree of immune suppression prevents adequate immune response or if the individual has already been exposed to both HPV-a and HPV-b. These individuals may still benefit from vaccination against HPV types to which they have not yet been exposed and to a lesser extent may also potentially benefit from reduction to reactivation of those HPV types with which they have previously been infected [54].
Efficacy of vaccine
Human papillomavirus vaccination was introduced into the routine immunization schedule in the United States in late 2006 for females before their first sexual contact and catch-up vaccination. The three dose series coverage was only 32% in 2010 and concomitantly the prevalence of HPV was reduced, which is the first measures of vaccine impact. Among females aged 14–19 years, there was a decreased HPV prevalence from 11.5% in 2003–2006 to 5.1% in 2007–2010, a decline of 56% indicating the estimated vaccine effectiveness is high [55].
High-coverage HPV vaccination programs among adolescents and young women may result in a rapid reduction of genital warts, cervical cytological abnormalities, and diagnostic and therapeutic procedures. In the longer term, substantial reductions in the rates of cervical, vulvar, and vaginal cancers may follow [56]. The efficacy of quadrivalent vaccine in Japanese women aged 18-26 years were assessed for 23 months. The vaccine has significantly reduce the acquisition of infection and clinical disease caused by HPV types 6, 11, 16 and 18 [57]. Similar efficacy analysis for HPV4 vaccine from eighteen countries showed 90.4% for HPV types of the vaccine cover [58]. Efficacy study on bivalent vaccine was also conducted in US among young women aged 15 through 25 years. Those women had not been exposed to a targeted HPV type before; the result demonstrated more than 99% of females developed an HPV 16 and 18 antibody response 1 month after completing the 3-dose series [59].
Conclusion and Recommendation
Virologic synergy between HIV and HPV infections further exacerbates the problem, and HIV-infected women are at increased risk for HPV and HPV-related diseases, including cervical cancer. There is no treatment option directed against HPV rather the related lesion, the natural immune responses could eliminate around 90% of HPV associated infectious cases. However, HPV has a wide range of immune evading mechanism. The HPV vaccines are highly immunogenic and have the ability to circumvent the viral immune evading mechanisms. The vaccine has no serious side effects, targeted for reproductive age groups, highly efficacious in preventing new infections of most common oncogenic HPV types and will dramatically reduce the rates of HPV associated cancer together with regular cervical screening.
There for:-
-The role of HIV infection on HPV will need to be carefully evaluated and strategies to maintain high levels of population coverage in vaccinated women need to be considered.
-Feature researches should be focus on the introduction of HPV vaccines in to routine immunization.
References
- Shailja P, Neeraj J, Bhupesh K, Suresh Bhambhani, Sanjay Gupta, Rajyashri Sharma, et al. Human Papillomavirus Type 16 Variant Analysis of E6, E7, and L1 Genes and Long Control Region in Biopsy Samples from Cervical Cancer Patients in North India. JCM. 2008; 46: 1060-1066.
- Gagnon S, Hankins C, Tremblay C, Forest P, Pourreaux K, Coutlée F. Canadian Women’s HIV Study Group . Viral polymorphism in human papillomavirus types 33 and 35 and persistent and transient infection in the genital tract of women. J Infect Dis. 2004; 190: 1575-1585.
- Trottier H, Franco EL. The epidemiology of genital human papillomavirus infection. Vaccine. 2006; 24: 1-15.
- Bosch F, Sanjose S. Human papillomavirus and cervical cancer-burden and assessment causality. J Natl Cancer Inst Monogr. 2003; 31: 3-13.
- Kravchenko J, Akushevich I, Sudenga L, Wilson CM, Levitan EB, Shrestha S. Transitional Probability Based Model for HPV Clearance in HIV-1-Positive Adolescent Females. J Clin Microbio. 2012; 7: 30736.
- Abraham AG, D’Souza G, Jing Y, Gange SJ, Sterling TR, Silverberg MJ, et al. Invasive cervical cancer risk among HIV-infected women: a North American multicohort collaboration prospective study. J Acquir Immune Defic Syndr. 2013; 62: 405-413.
- Elfstrom K, Herweijer E, Sundström K, Arnheim-Dahlström L. Current cervical cancer prevention strategies including cervical screening and prophylactic human papillomavirus vaccination. J Curr Opin Oncol. 2014; 26: 120-129.
- Palefsky J. HPV infection and HPV-associated neoplasia in immunocompromised women. Int J Gynaecol Obstet. 2006; 94: 56-64.
- Anastos K, Hoover DR, Burk RD, Cajigas A, Shi Q, Singh DK, et al. Risk factors for cervical precancer and cancer in HIV-infected, HPV-positive Rwandan women. PLoS One. 2010; 5: 13525.
- Oster AM, Sullivan PS, Blair JM. Prevalence of cervical cancer screening of HIV-infected women in the United States. J Acquir Immune Defic Syndr. 2009; 51: 430-436.
- Franco EL, Villa LL, Sobrinho JP, Prado JM, Rousseau MC, Désy M, et al. Epidemiology of acquisition and clearance of cervical human papillomavirus infection in women from a high-risk area for cervical cancer. J Infect Dis. 1999; 180: 1415-1423.
- Dunne EF, Unger ER, Sternberg M, McQuillan G, Swan DC, Patel SS, et al. Prevalence of HPV infection among females in the United States. JAMA. 2007; 297: 813-819.
- Hariri S, Unger ER, Sternberg M, Dunne EF, Swan D, Patel S, et al. Prevalence of genital human papillomavirus among females in the United States, the National Health And Nutrition Examination Survey, 2003-2006. J Infect Dis. 2011; 204: 566-573.
- Gillison ML, Broutian T, Pickard RK, Tong ZY, Xiao W, Kahle L, et al. Prevalence of oral HPV infection in the United States, 2009-2010. JAMA. 2012; 307: 693-703.
- Doorbar J. The papillomavirus life cycle. J Clin Virol. 2005; 32: 7-15.
- Wentzensen N, Schiffman M, Dunn S, Zuna RE, Walker J, Allen RA, et al. Grading the severity of cervical neoplasia based on combined histopathology, cytopathology, and HPV genotype distribution among 1,700 women referred to colposcopy in Oklahoma. Int J Cancer. 2009; 124: 964-969.
- Marc A, Frank B, Marc V, Evangelos P, Pierre M, Joakim D. A randomized trial on the management of low-grade squamous intraepithelial lesion cytology interpretations. Am J Obstet Gynecol. 2003; 188: 1393–1400.
- AbdullGaffar B, Kamal MO, Hasoub A. The prevalence of abnormal cervical cytology in women with infertility. Diagn Cytopathol. 2010; 38: 791-794.
- Dunne EF, Unger ER, Sternberg M, McQuillan G, Swan DC, Patel SS, et al. Prevalence of HPV infection among females in the United States. JAMA. 2007; 297: 813-819.
- Getahun F, Mazengia F, Abuhay M, Birhanu Z. Comprehensive knowledge about cervical cancer is low among women in Northwest Ethiopia. BMC Cancer. 2013; 13: 2.
- Hailu A, Mariam DH. Patient side cost and its predictors for cervical cancer in Ethiopia: a cross sectional hospital based study. BMC Cancer. 2013; 13: 69.
- Mayrand MH, Duarte-Franco E, Rodrigues I, Walter SD, Hanley J, Ferenczy A, et al. Human papillomavirus DNA versus Papanicolaou screening tests for cervical cancer. N Engl J Med. 2007; 357: 1579-1588.
- Ho GY, Studentsov YY, Bierman R, Burk RD. Natural history of human papillomavirus type 16 virus-like particle antibodies in young women. Cancer Epidemiol Biomarkers Prev. 2004; 13: 110-116.
- Eduardo L, Eliane D, Alex F. Cervical cancer: epidemiology, prevention and the role of human papillomavirus infection. CMAJ. 2001; 164: 1017–1025.
- Bryan T. New HPV Vaccine Recommendations Could Have Multiple Benefits. J OncoLog. 2012; 57: 3-9.
- Herrero R, Hildesheim A, Bratti C, Sherman ME, Hutchinson M, Morales J, et al. Population-based study of human papillomavirus infection and cervical neoplasia in rural Costa Rica. J Natl Cancer Inst. 2000; 92: 464-474.
- Bontkes H, Gruijl T, Walboomers J, Schiller JT, Dillner J, Helmerhorst TJ, et al. Immune responses against HPV type 16 virus like particles in a cohort study of women with cervical intraepithelial neoplasia. but not local IgA responses correlate with clearance of HPV–16. J Gen Virol.1999; 80: 409– 417.
- Amador-Molina A, Hernández-Valencia JF, Lamoyi E, Contreras-Paredes A, Lizano M. Role of innate immunity against human papillomavirus (HPV) infections and effect of adjuvants in promoting specific immune response. Viruses. 2013; 5: 2624-2642.
- Stanley MA. Immune responses to human papilloma viruses. Indian J Med Res. 2009; 130: 266-276.
- Karim R, Tummers B, Meyers C, Biryukov JL, Alam S, Backendorf C, et al. Human papillomavirus (HPV) upregulates the cellular deubiquitinase UCHL1 to suppress the keratinocyte’s innate immune response. PLoS Pathog. 2013; 9: 1003384.
- Margaret S. Human Papillomavirus immune response to infection and vaccination. J Iinf agen canc. 2010; 5: 19.
- Colmenares V, Noyola E, Monsiváis A, Salgado-Bustamante M, Estrada- Capetillo L, González-Amaro R, et al. Human Papillomavirus Immunization Is Associated with Increased Expression of Different Innate Immune Regulatory Receptors. J ASM. 2013; 20: 12.
- Romanowski B. Long term protection against cervical infection with the human papillomavirus: review of currently available vaccines. J Human Vaccines. 2011; 7: 161–169.
- Hughes JP, Garnett GP, Koutsky L. The theoretical population-level impact of a prophylactic human papilloma virus vaccine. Epidemiology. 2002; 13: 631-639.
- Human Papillomavirus 9-valent Vaccine. Recombinant Suspension for intramuscular injection Initial U.S. Approval. 2014.
- Zurkova K, Babiarova K, Hainz P, Krystofova J, Kutinova L, Otahal P, et al. The expression of the soluble isoform of hFlt3 ligand by recombinant vaccinia virus enhances immunogenicity of the vector. Oncol Rep. 2009; 21: 1335- 1343.
- Lee W, Anderson E, Wu S, Lee H. Development of an adenoviral vaccine against E6 and E7 oncoproteins to prevent growth of HPV and Therapeutic Vaccines of human papillomavirus-positive cancer. Arch Otolaryngol Head Neck Surg. 2008; 134: 1316-1323.
- Colgrove J, Abiola S, Mello MM. HPV vaccination mandates--lawmaking amid political and scientific controversy. N Engl J Med. 2010; 363: 785-791.
- Garnett GP. Role of herd immunity in determining the effect of vaccines against sexually transmitted disease. J Infect Dis. 2005; 191: 97-106.
- Lauri E, Susan H, Carol L, Eileen F, Martin S, Geraldine M, et al. Reduction in Human Papillomavirus (HPV) Prevalence Among YoungWomen Following HPV Vaccine Introduction in the United States, National Health and Nutrition Examination Surveys, 2003–2010. JID. 2013; 208: 385-393.
- Paavonen J, Naud P, Salmeron J, Wheeler CM, Chow SN, Apter D, et al. Efficacy of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA): final analysis of a double-blind, randomised study in young women. Am J Public Health. 2009; 374: 301–314.
- Mattarollo SR, Yong M, Gosmann C, Choyce A, Chan D, Leggatt GR, et al. NKT cells inhibit antigen-specific effector CD8 T cell induction to skin viral proteins. J Immunol. 2011; 187: 1601-1608.
- Franco EL, Harper DM. Vaccination against human papillomavirus infection: a new paradigm in cervical cancer control. Vaccine. 2005; 23: 2388-2394.
- Paavonen J, Jenkins D, Bosch F, Naud P, Salmerón J, Wheeler C, et al. Efficacy of a prophylactic adjuvanted bivalent L1 virus-like-particle vaccine against infection with human papillomavirus types 16 and 18 in young women: an interim analysis of a phase III double-blind, randomised controlled trial. J New En M. 2007; 369: 2161-2170.
- Bosch F, Tsu V, Vorsters A, Van Damme P, Kane MA. Reframing cervical cancer prevention. Expanding the field towards prevention of human papillomavirus infections and related diseases. JVacc. 2012; 20: 1-11.
- Kim S, Jeong H, Park S, Kim HJ. Purification and immunogenicity study of human papillomavirus type 16 L1 protein in Saccharomyces cerevisiae. J Virol Methods. 2007; 139: 24-30.
- Lobermann M, Borso D, Hilgendorf I, Fritzsche C, Zettl UK, Reisinger EC. Immunization in the adult immunocompromised host. Autoimmun Rev. 2012; 11: 212-218.
- Garçon N, Wettendorff M, Van Mechelen M. Role of AS04 in human papillomavirus vaccine: mode of action and clinical profile. Expert Opin Biol Ther. 2011; 11: 667-677.
- Gold MS, Buttery J, McIntyre P. Human papillomavirus vaccine safety in Australia: experience to date and issues for surveillance. J Sex Healt. 2010; 7: 320-324.
- Mira C Ketaki K, Atanu B, Mohandas KM, Mukhopadhyaya R. Production of immunogenic human papillomavirus-16 major capsid protein derived virus like particles. Indian J Med Res. 2009; 130: 213-218.
- Averbach S, Gravitt P, Nowak R, Celentano DD, Dunbar MS, Morrison CS, et al. The association between cervical human papillomavirus infection and HIV acquisition among women in Zimbabwe. JAIDS. 2010; 24: 1035–1042.
- Auvert B, Lissouba P, Cutler E, Zarca K, Puren A, Taljaard D. Association of oncogenic and nononcogenic human papillomavirus with HIV incidence. J Acquir Immune Defic Syndr. 2010; 53: 111-116.
- Denny L, Boa R, Williamson AL, Allan B, Hardie D, Stan R, et al. Human papillomavirus infection and cervical disease in human immunodeficiency virus-1-infected women. Obstet Gynecol. 2008; 111: 1380-1387.
- Palefsky JM, Gillison ML, Strickler HD. Chapter 16: HPV vaccines in immunocompromised women and men. Vaccine. 2006; 24: 140-146.
- Lauri E, Susan H, Carol L. Reduction in Human Papillomavirus (HPV) Prevalence Among Young Women Following HPV Vaccine Introduction in the United States. JID. 2013; 1: 1-9.
- Muoz N, Kjaer SK, Sigurdsson K, Iversen OE, Hernandez-Avila M, Wheeler CM, et al. Impact of human papillomavirus (HPV)-6/11/16/18 vaccine on all HPV-associated genital diseases in young women. J Natl Cancer Inst. 2010; 102: 325-339.
- Yoshikawa H, Ebihara K, Tanaka Y, Noda K. Efficacy of quadrivalent human papillomavirus (types 6, 11, 16 and 18) vaccine (GARDASIL) in Japanese women aged 18-26 years. Cancer Sci. 2013; 104: 465-472.
- Giuliano AR, Palefsky JM, Goldstone S, Moreira ED Jr, Penny ME, Aranda C, et al. Efficacy of quadrivalent HPV vaccine against HPV Infection and disease in males. N Engl J Med. 2011; 364: 401-411.
- Einstein M, Baron M, Levin M, Chatterjee A, Edwards RP, Zepp F, et al. Comparison of the immunogenicity and safety of Cervarix and Gardasil human papillomavirus (HPV) cervical cancer vaccines in healthy women aged 18-45 years. J Hum Vaccin. 2009; 5: 705–719.