
Editorial
Austin J Clin Immunol. 2016; 3(1):1028.
Mannose-6-Phosphatereceptor may be a Novel Checkpoint for T Cell Expansion in HIV+ Patients
Christof Tobias Kaltenmeier, Hubert Schrezenmeier and Bernd Jahrsdörfer*
Institute of Clinical Transfusion Medicine and Immunogenetics, Germany
*Corresponding author: Bernd Jahrsdörfer, Laboratory of Cellular Immunology, Institute of Clinical Transfusion Medicine and Immunogenetics, Helmholtzstrasse 10, 89081 Ulm, Germany
Received: February 23, 2016; Accepted: February 23, 2016; Published: March 01, 2016
Editorial
Recently, we demonstrated that untreated patients with HIV infection display high peripheral blood counts of regulatory B cells expressing the serine protease Granzyme B (GrB) in the absence of perforin (GraB cells) [1]. Importantly, these so-called GraB cells are able to directly regulate proliferation and survival of T cells both in-vitro and in-vivo. The mechanism of action involves a perforin-independent transfer of GrBto T cells and GrB-dependent degradation of the T cell receptor ζ-chain in T cells [1,2].
A known receptor for GrB, which acts in a perforin-independent manner, is the mannose-6-phosphate receptor (M6PR, CD222), which has been shown to mediate GrB uptake and regulation of M6PRexpressing target cells [3,4]. A recent study in Listeria-infected mice demonstrated that the differential expression of M6PRon cytotoxic T cells is directly linked to their survival and proliferative capacity [5]. M6PR therefore appears to represent an important check point for T cell expansion and memory T cell formation after systemic infections.
Here we report our very recent findings confirming this mechanism in human patients with untreated HIV infections. Since cellular uptake of GrB in the absence of perforincan occur in an M6PR-dependent manner [3,4], we tested the expression of M6PR on T cells from untreated HIV patients and compared it to healthy controls. These experiments revealed that M6PR expression by T cells from HIV patients is significantly higher than by T cells from healthy control subjects (Figure 1). Our data therefore suggest that defects in the memory T cell compartment in HIV patients may at least in part be due to elevated expression of M6PR by T cells, associated with a higher sensitivity of these cells to GrB-mediated apoptosis and growth arrest.
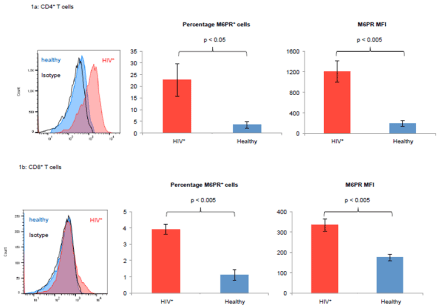
Figure 1: T cells from untreated HIV+ patients express high levels of M6PR as compared with T cells from healthy individuals. PBMC from 4 untreated HIV patients
and from 4 healthy control subjects were isolated and stained with fluorescently labeled antibodies against CD3, CD4, CD8 and M6PR (CD222). Subsequently,
CD4+ (Figure 1a) and CD8+ (Figure 1b) T cells were analyzed by flow cytometry. Histograms show M6PR surface expression from one representative experiment
(left panels). Bar graphs show average percentages of M6PR+ T cells (middle panels) and M6PR median fluorescence intensity (MFI) values (right panels). Error
bars indicate SEM, *indicates p < 0.05, **indicates p < 0.005.
In summary, our findings support the current view that after infections with intracellular pathogens such as viruses or intracellular bacteria, activated T cells differentially regulate M6PR on their cell surface [5]. Such differential expression of M6PR on T cells may not only explain how regulatory T cells may initiate the effector T cell contraction phase after an infection, but also how other immune cell populations expressing GrB in the absence of perforin such as plasmacytoid dendritic cells or GraB cells [2,6,7] may directly regulate T cell expansion in an M6PR- and GrB-dependent fashion (Figure 2). Modulation of M6PR on T cells by pharmacological means could therefore represent a promising novel approach to enhance or suppress T cell-mediated immunity in different infectious diseases including HIV infection.
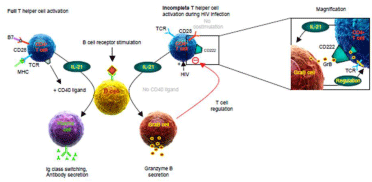
Figure 2: Mannose-6-phosphate receptor (M6PR, CD222) on T cellsfrom HIV patients mediates their suppression by granzyme B-secretingregulatory B cells
(GraB cells). During HIV infection, the T Cellreceptor (TCR) of CD4+ T cells is directly stimulated via the HIV protein Nef, without simultaneousc ostimulation of
CD28. In contrast to fully activated T cells (left panelside), such incompletely activated T cells secrete IL-21, but barely express CD40L, resulting in the induction
of granzyme B-secreting GraB cells instead of plasmacells (rightpanelside, Copyright 2015. The American Association of Immunologists, Inc). By concomitant
upregulation of CD222 on T cells in the course of an HIV infection, the cellular uptake of exogenous granzyme B by T cells is strongly enhanced, resulting in
increased cleavage of their TCR ζ-chain (magnificationpanel). Lower TCR ζ-chain levels are associated with lower proliferative capacity of such T cells. Breaking
of this vicious circle maybe possible by exogenous addition of CD40L multimers, which can suppress the generation of GraBcells after incomplete B cell/T cell
interactions during HIV infection.
References
- Kaltenmeier C, Gawanbacht A, Beyer T, Lindner S, Trzaska T, van der Merwe JA, et al. CD4+ T cell-derived IL-21 and deprivation of CD40 signaling favor the in vivo development of granzyme B-expressing regulatory B cells in HIV patients. Journal of immunology. 2015; 194: 3768-3777.
- Lindner S, Dahlke K, Sontheimer K, Hagn M, Kaltenmeier C, Barth TF, et al. Interleukin 21-induced granzyme B-expressing B cells infiltrate tumors and regulate T cells. Cancer Res. 2013; 73: 2468-2479.
- Motyka B, Korbutt G, Pinkoski MJ, Heibein JA, Caputo A, Hobman M, et al. Mannose 6-phosphate/insulin-like growth factor II receptor is a death receptor for granzyme B during cytotoxic T cell-induced apoptosis. Cell. 2000; 103: 491-500.
- Veugelers K, Motyka B, Goping IS, Shostak I, Sawchuk T, Bleackley RC. Granule-mediated killing by granzyme B and perforin requires a mannose 6-phosphate receptor and is augmented by cell surface heparan sulfate. Mol Biol Cell. 2006; 17: 623-633.
- Ahmed KA, Wang L, Griebel P, Mousseau DD, Xiang J. Differential expression of mannose-6-phosphate receptor regulates T cell contraction. J Leukoc Biol. 2015; 98: 313-318.
- Jahrsdörfer B, Vollmer A, Blackwell SE, Maier J, Sontheimer K, Beyer T, et al. Granzyme B produced by human plasmacytoid dendritic cells suppresses T-cell expansion. Blood. 2010; 115: 1156-1165.
- Hagn M, Sontheimer K, Dahlke K, Brueggemann S, Kaltenmeier C, Beyer T, et al. Human B cells differentiate into granzyme B-secreting cytotoxic B lymphocytes upon incomplete T-cell help. Immunol Cell Biol. 2012; 90: 457- 467.