
Research Article
Austin J Clin Immunol. 2016; 3(1):1030.
IL-27 Concentration in Systemic Circulation and Tumor Micro-Circulation Samples of Sclc and Nsclc Patients; Association with Tumor Size, Histological Type and Presence of Metastases
Karlicic V¹, Vukovic J¹, Stanojevic I2,9, Sotirovic J3, Peric A3, Jovic M4, Cvijanovic V5, Djukic M6, Banovic T7, Pantic Bisevac J8 and Vojvodic Danilo2,9*
¹Clinic for Lung Disease, Military Medical Academy, Serbia1Clinic for Lung Disease, Military Medical Academy, Serbia
²Institute for Medical Research Military Medical Academy, Serbia
³Clinic for Ear, Nose and Throat, Military Medical Academy, Serbia
4Institute of Pathology and Forensic Medicine Military Medical Academy, Serbia
5Clinic for Thoracic Surgery, Military Medical Academy, Serbia
6Department of Toxicology, Belgrade University, Serbia
7Department of Immunology, Australia
8Institute for Medical Biochemistry, Military Medical Academy, Serbia
9Medical Faculty, University of Defense, Serbia
*Corresponding author: Vojvodic D, Institute for Medical Research, Military Medical Academy, Medical Faculty, University of Defense, Crnotravska 17, 11000 Belgrade, Serbia
Received: July 08, 2016; Accepted: August 05, 2016; Published: August 09, 2016
Abstract
IL-27 Concentration in systemic circulation and tumor micro-circulation samples of sclc and nsclc patients; association with tumor size, histological type and presence of metastases
Introduction: Advanced lung carcinoma is charasterized with fast disease progression. IL-27 is considered as a cytokine with anti-tumor activities and its role in lung carcinoma pathogenesis and in the anti-tumor response has not yet been elucidated.
Aim: To correlate IL-27 concentration in serum and lung tumor microcirculation with clinical stage, disease outspread, pathohistological features and TNM stadium.
Patients and Methods: The study included 41 lung tumor patients in III and IV clinical stage. Histological type was determined immunohistochemically, while tumor size, localization and dissemination were determined radiologically (MSCT). IL-27 concentration was quantified with commercial ELISA test in serum and lung tumor microcirculation samples.
Results: IL-27 concentration was significantly decreased in samples of all patients comparing to controls. In the patient’s samples, IL-27 was significantly increased in tumor microcirculation comparing to sera samples. Patients with NSCLC large cell histology type had the lowest IL-27 concentration, both in tumors and sera samples. Degree of disease spread was significantly associated with IL-27 levels, because concentration was significantly decreased in patient’s clinical stage IV or those with metastases, in both types of samples. Both in tumor microcirculation and sera samples, there was significant association of tumor size and IL-27 concentration, with the highest value of IL-27 in patients with the smallest lung tumors.
Conclusion: IL-27 concentration is significantly elevated in tumor microenvironment of investigated lung cancer patients. IL-27 values significantly differ between lung cancer patients according to tumor histology, degree of disease extent and size of tumor. Significant association of higher IL-27 concentration with smaller tumors, earlier clinical stage and absence of metastases indicates that IL-27 cytokine have anti-tumor functions in these patients.
Keywords: NSCLC; SCLC; IL-27; Serum; Tumor microcirculation; Tumor size
Abbreviations
SCLC: Small Cell Lung Cancer; NSCLC: Non Small Cell Lung Cancer; NSCLC Ad: NSCLC Adenocarcinoma; NSCLC Sq: NSCLC Squamous Carcinoma; NSCLC LC: NSCLC Large Cell Carcinoma; EBI3: Epstein Barr Induced 3 gene
Introduction
Interleukin 27 is a relatively recently characterized cytokine mainly recognized by its anti tumor effects [1]. By structure it belongs to IL-12 cytokine family that includes heterodimeric cytokines IL- 12 (composed of IL-12p35 + IL-12p40), IL-23 (composed of IL- 23p19 + IL-12p40), IL-27 (composed of IL-27p28 + EBI3) and IL-35 (composed of IL-12p35 + EBI3) [2]. IL-27 exerts its effect by binding specific IL-27 receptor constructed of two subunits, WSX and gp130 [3]. Unique characteristic of this receptor that every subunit is bound to a distinct transcription factor (WSX1 subunit to STAT1 and gp130 subunit to STAT3) so IL-27 have capacity to efficiently activate both STAT1 and STAT3 cell transcription factors. First demonstrated function of IL-27 was its inflammatory role, based on study where mice deficient for alpha subunit of IL27R showed significant susceptibility for L. Monocytogenes and L. Mayor Infections [4]. Pflanz et al. also showed that IL-27 supported proliferation and stimulation of IFN-γ secretion from CD4+T lymphocytes. IL-27 induces the TH1 T cells expansion [5] and inhibits differentiation of inducible regulatory T lymphocytes [6]. Beyond CD4+T lymphocytes IL-27 stimulates proliferation and the expression of IFN-γ and beta subunit of IL12 on CD8+T lymphocytes [7]. IL-27 potently affect Cytotoxic T Lymphocyte response (CTL) by inducing the expression of granzyme B [8,9].
Biological functions of IL-27 are mainly characterized from studies in different tumor models, where IL-27 has demonstrated antitumor activities. IL-27 suppressed tumor growth in metastatic murine neuroblastoma tumors [10], murine colon carcinoma cells [11,12], melanoma cell lines [13,14], head and neck squamous cell carcinoma [15], lung carcinoma cell lines [16] and multiple myeloma [17]. Some of this anti tumor effects were mediated by CD8+ T lymphocytes, as shown in models of metastatic murine neuroblastoma tumors [10] and murine colon carcinoma cells [11]. In other tumor models IL-27 acted through different mechanisms, enhanced NK cell anti tumor activity [13], inhibited angiogenesis [12], directly suppressed tumor cell proliferation [14,17] and induced antibody dependent tumor cell cytotoxicity [15]. Data from all these studies demonstrated anti tumor effects of IL-27, executed through various effector mechanisms.
But not all biological activities of IL-27 are stimulatory for immune cells. IL-27 inhibits the development of Th17 T lymphocytes and induces IL-10 secretion [18]. In some conditions IL-27 is an important factor for differentiation of induced regulatory T lymphocytes (Treg) with potent IL-10 producing capacity [19-22], and transforms dendritic cells into immunosuppressive by induction of B7-H1 expression [23]. In vitro study in a model of a human epithelial ovarian cells additionally demonstrated that IL-27 induced expression of IL18 inhibitor, together with Programmed Death Ligand -1 (PD-1L) and Indoleamine 2,3 Dioxigenase (IDO), potent immunosuppressive check points in activation of adaptive immune response [1].
Studies that analyze IL-27 significance in lung cancer patients are still rare [24-27]. We have shown that high IL-27 sera levels are associated with early clinical stage and limited disease in patients with melanoma [28]. However, because still little is known about IL-27 importance in lung cancer patients, in this study we have investigated association of IL-27 levels in systemic circulation and tumor microcirculation with clinical parameters and tumor characteristics in our SCLC and NSCLC patients.
Material and Methods
Patients
The study included 41 patients, diagnosed for lung cancer and 30 healthy controls (Table 1). Patients were diagnosed and treated at Clinic for lung diseases, Military Medical Academy, Belgrade, Serbia, in period from March till December 2015. All necessary diagnostic procedures (histological, laboratory and radiological) were performed at Military Medical Academy, Belgrade, Serbia. All patients and healthy controls were consented and this study was approved by the local Research Ethics Committee, Military Medical Academy (11-03/2014).
Samples
Blood samples were taken from cubital vein. Tumor microcirculation samples were taken from accessible pathological blood vessels with needle aspiration during diagnostic bronchoscopy. Serum was separated from the samples after centrifugation (3000g, 10 minutes, RT) and frozen at -70 °C until testing. IL-27 was quantified with commercial ELISA test (R&D Systems).
Statistical analysis
All statistical tests were performed with software GraphPad Prism 5, using ANOVA test (with Bonferroni post testing) for multiple group comparisons, Mann Whitney test for to compare differences between two independent groups, Wilcoxon for comparison of paired (serum/tumor) samples and Pearson correlation test.
Results
IL-27 in control and lung cancer patient samples
Healthy control persons had significantly higher average IL- 27 concentration comparing to lung cancer patients (Table 1). This finding was constant whether we are analyzed all subjects or after we divided patients according to sex. Healthy men demonstrated higher average IL-27 concentration comparing to healthy women. This difference was not as pronounced in sera samples from lung cancer patients of different sex. Analysis of tumor microcirculation samples showed higher average IL-27 value in samples of women with lung tumors.
Lung cancer histology and IL-27 concentration
The lowest average serum IL-27 concentration was detected in samples of patients that had NSCLC Large Cell histology tumors, significantly less than all other investigated types (Table 1& Figure 1). The same relationship was found in the tumor microcirculation samples, again with the lowest average IL-27 concentration in same patient group. All tumor samples had increased IL-27 level comparing to corresponding sera samples, with the significant increase in tumor compartment in patients with SCLC and NSCLC adenocarcinoma tumors.
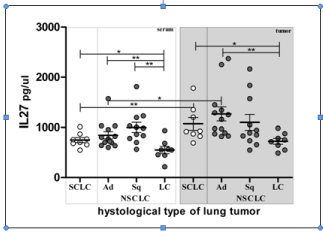
Figure 1: Lung cancer histology and IL-27 concentration. Individual values
are shown as circles. Bar and lines represesent the mean IL-27 value ± SD,
pg/ml. Asterisk represent statisticaly significant difference (*p<0.05, **p<0.01,
***p<0.001, Mann Whintey test).
Higher IL-27 concentration in patients with clinical stage III
Patients that were in III clinical stage had increased average IL- 27 concentration comparing to patients clinical stage IV, in serum and tumor microcirculation (Table 1& Figure 2). This increment was only significant in comparison of serum samples. Again, all tumor samples had increased IL-27 level comparing to corresponding sera samples.
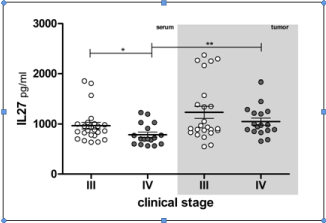
Figure 2: IL-27 concentration in lung cancer patients of III and IV clinical
stage. Individual values are shown as circles. Bar and lines represesent
the mean IL-27 value ± SD, pg/ml. Asterisk represent statisticaly significant
difference (*p<0.05, ** p<0.01, ***p<0.001, Mann Whintey test).
Absence of metastases is associated with higher IL-27
Lung cancer patients without metastases had significantly increased average IL-27 concentration comparing to patients with metastatic disease, both in serum and tumor microcirculation (Table 1& Figure 3). Average IL-27 concentration samples both from M0 and M1 patients groups had significantly more IL-27 than corresponding sera samples.
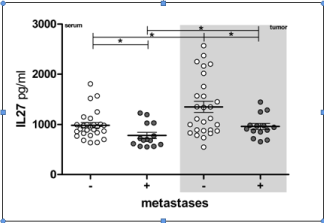
Figure 3: IL-27 concentration in lung cancer patients with or without
metasases. Individual values are shown as circles. Bar and lines represesent
the mean IL-27 value ± SD, pg/ml. Asterisk represent statisticaly significant
difference (*p<0.05, **p<0.01, *** p<0.001, Mann Whintey test).
Tumor size and IL-27 concentration
We found clear association of tumor size with IL-27 concentration, both in serum and tumor samples. Patients with the smallest Tumors (T1) had significantly increased IL-27 concentration comparing to all other groups, both in serum ant tumor microcirculation samples (Table 1 & Figure 4). In other words, increase of tumor size is inversely associated with IL-27 concentration, in both compartments, systemic circulation and tumor micro-environment.
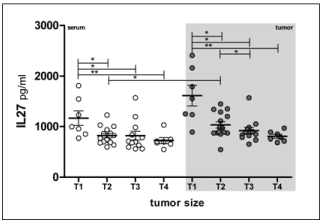
Figure 4: Tumor size and IL-27 concentration. Individual values are shown as
circles. Bar and lines represesent the mean IL-27 value ± SD, pg/ml. Asterisk
represent statisticaly significant difference (*p<0.05, **p<0.01, ***p<0.001,
Mann Whintey test).
Discussion
The earliest information about anti tumor activity of IL-27 have been presented by Ho et al., who established specific model with genetically manipulated murine Lewis lung carcinoma cell line that produced IL-27 [16]. They showed that over expression of transduced IL-27 potently inhibited growth of lung cancer cells in vitro. Growth retardation was accompanied with reduction of invasive capacity (decrement of vimentin and increment of cadherin), together with down regulation of COX-2, PGE2 and TGFβ1 production from cancer cells. In vivo experiments demonstrated that such IL-27 transduced lung cancer cells stimulated a specific anti tumor response mediated by CTL of Th1 type. Finally, they showed that recombinant IL-27 negatively regulated PGE2, COX-2 and vimentin expression in human NSCLC cancer cell lines. Authors concluded that IL-27 have numerous and various anti tumor activities, making it attractive as therapy agent for lung cancer.
Naumnik et al. investigated significance of IL-27, IL-29, IL-31 and IL-39 in serum and BAL samples of patients with advanced lung cancer (only NSCLC type) [24]. They showed that average IL-27 value did not differ significantly between lung cancer patients and patients with non malignant inflammatory lung diseases (sarcoidosis, allergic alveolitis), but were significantly higher comparing to healthy controls. Interestingly, they found that sera samples of all investigated groups contained significantly more IL-27 than BAL samples. This is in contrast with our data, since we demonstrated that all tumor samples had more IL-27 comparing to corresponding serum samples. Authors discussed this difference as a consequence of decrepit local anti tumor response comparing to systemic, or because of its origin, since it is produced and released from endothelial cells and hematopoietic cells. We believe that explanation is in nature of sample, because tumor microcirculation represent direct tumor microenvironment, while BAL sample is diluted washing of distant tumor. Naumnik et al. further demonstrated insignificant IL- 27 increase in serum samples of patients stage IIIB over patients stage IV, as well as increase of BAL IL-27 after chemotherapy in patients with partial remission comparing to patients with stabile disease.
Barrera et al. studied association of cytokine profile with survival prognosis in serum samples 110 NSCLC patients [25]. Among investigated cytokines (IL-1β, IL-2, IL-6, IL-8, IL-12, IL-17, IFN-γ, TNF, IL-4, IL-10, IL-31, IL-33, IL-27, IL-29), IL-27 concentration solely was not significantly associated with any group of overall survival that they defined. IL-27 was significantly higher in non smoking patients, and insignificantly increased in female NSCLC patients, those patients that were IIIB stage, patients that had no CNS metastases, in patients with >20 kg/m2 body mass index value and in patients with positive pleural effusion. These authors also demonstrated that finding of IL4 value above 0.9 pg/ml was significantly associated with higher IL-27 levels and this cytokine cluster was characteristic for patients with better prognosis. Contrary, patients that had cluster of low IL-12, IL-6, IL-27 and IL-29 together with high IL-4 and IL-8 belonged to the group with worse survival prognosis. With the exception of IL- 6, these data indicate that decreased values of IL-12 cytokine family are associated with worse prognosis in NSCLC patients.
With aim to enlight inflammatory process in NSCLC pathogenesis, Duan et al. investigated IL-17 and IL-27 values in group of 19 NSCLC patients [26]. They demonstrated significant decrement of IL-27 in NSCLC patients comparing to healthy controls. This decrease was evident both on protein (serum samples) and mRNA level (peripheral blood cells).
Li et al. investigated significance of IL-27 in lung cancer patients that suffer from pleural effusion with presence of malignant cells [27]. They showed that local micorenvironment strongly affects IL- 27 production, demonstrating that malignant pleural effusion from lung cancer patients contained more IL-27 and higher number of IL-27+CD4T lymphocytes than corresponding samples of peripheral blood. In vitro conditions they also demonstrated that IL-27 significantly phosphorylated STAT1 and STAT3, inhibited proliferation of A549 cell line by downregulated expression of Ki67, inhibited cell migratory in response to pleural mesothelial cells and induced expression of adhesive molecules ICAM1, VCAM1 and LFA1 both on A549 cell line and pleural mesothelial cells. They concluded that locally produced IL-27 exerted important anti tumor functions.
Studies of JM Lee group [29,30] provided explanation for molecular mechanism of IL-27 anti-tumor action in lung cancer. They exposed eight lung cancer lines from NSCLC patients to 24 hour activity of IL-27. With exception of two tumor cell lines, all other responded with significant increase of STAT1 and STAT3 upon IL-27 stimulation [29]. This increase of STAT1 and STAT3 was characterized with its translocation into nuclear compartment of NSCLC lines, followed by increased expression of epithelial markers (E-cadherin and γ-catenin), epithelial phenotype acquisition of lung cancer cells, inhibition of in vitro cell migration and inhibition of angiogenic factor production (down regulation of VEGF, IL-8/CXCL8 and CXCL5). This indirect anti angiogenic activity of IL-27 has been already demonstrated before, in different tumor model, myeloma cell lines [17]. Addition of selective COX-2 inhibitor Apricoxib had more dramatic effect on inhibition of process epithelial to mesenchymal transition, which is crucial step in metastasis [30].
Significant contribution in understanding of IL-27 mechanism of action in the pathogenesis of lung carcinoma came from study of Airoldi et al. [31]. They investigated IL-27 activity in vitro (NSCLC and SCLC cell lines), in vivo (xenograft models) and also studied IL-27R expression in biopsy specimens of 78 lung cancer patients (NSCLC). They demonstrated that both type of lung cancer cell lines expressed IL-27R and responded to recombinant IL-27 with upregulation of CXCL-3 expression, and suppression of genes that control stemness (OCT4A, SRY -box 2, SOX2, SOX9 , NOTCH1, and KLF4) and epithelial to mesenchymal transition. These genetical changes were followed with morphological changes of lung cancer cells that showed transformation from fibroblast like cells to polygonal cells with expression of the adhesive E Cadherin, characteristic of less aggressive cell sub-types. In vivo, in xenograft lung cancer model, IL-27 significantly delayed or obstructed tumor growth together with impressive level of colliquative necrosis and apoptosis. This is very important, because they demonstrated that even in animal deficient with T or B lymphocytes, IL-27 activated myeloid cells could infiltrate and destroy tumor cells. Final proof was myeloablative treatment of these animals that prevented in vivo anti tumor effects of applied IL-27, and showed that granulocytes and macrophages are important for IL-27 anti lung cancer activity in vivo, at least in this model.
We have demonstrated that IL-27 concentration is significantly elevated in tumor microenvironment of investigated lung cancer patients comparing to corresponding sera samples. Although decreased comparing to healthy controls, patients with earlier clinical stage, smaller tumors and without metastases have significantly more IL-27 in both investigated compartments than those patients with large tumors and widespread disease. Histological type of tumor is important not only dye to specific biological characteristics, but also because of significant difference in IL-27 production. Significant association of higher IL-27 concentration with smaller tumors, earlier clinical stage and absence of metastases indicates that IL-27 cytokine have anti-tumor functions in lung cancer patients.
References
- Carbotti G, Barisione G, Airoldi I, Mezzanzanica D, Bagnoli M, Ferrero S, et al. IL-27 induces the expression of IDO and PD-L1 in human cancer cells. Oncotarget. 2015; 6: 43267-43280.
- Chen G, Liang Y, Guan X, Chen H, Liu Q, Lin B, et al. Circulating low IL-23: IL-35 cytokine ratio promotes progression associated with poor prognosisin breast cancer. Am J Transl Res. 2016; 8: 2255-2264.
- Yoshimoto T, Owaki T, Asakawa M, Morishima N, Kamiya S, Mizuguchi J. A novel IL-6/IL-12 family cytokine IL-27 and its antitumor activity. Gene Ther Mol Biol. 2005; 9: 7-14.
- Pflanz S, Timans JC, Cheung J, Rosales R, Kanzler H, Gilbert J, et al. IL- 27, a heterodimeric cytokine composed of Ebi3 and p28 protein induces proliferation of naive CD4+ T cells. Immunity. 2002; 16: 779–790.
- Takeda A, Hamano S, Yamanaka A, Hanada T, Ishibashi T, Mak, TW, et al. Role of IL-27/WSX-1 signaling for induction of T-bet through activation of STAT1 during initial Th1 commitment. J Immunol. 2003; 170: 4886–4890.
- Cox JH, Kljavin NM, Ramamoorthi N, Diehl L, Batten M, Ghilardi N. IL-27 promotes T cell-dependent colitis through multiple mechanisms. J Exp Med. 2011; 208: 115–123.
- Schneider R, Yaneva T, Beauseigle D, El-Khoury L, Arbour N. IL-27 increases the proliferation and effector functions of human naive CD8+ T lymphocytes and promotes their development into Tc1 cells. Eur J Immunol. 2011; 41: 47–59.
- Morishima N, Owaki T, Asakawa M, Kamiya S, Mizuguchi J, Yoshimoto T. Augmentation of effector CD8+ T cell generation with enhanced granzyme B expression by IL-27. J. Immunol. 2005; 175: 1686–1693.
- Morishima N, Mizoguchi I, Okumura M, Chiba Y, Xu M, Shimizu M, et al. A pivotal role for interleukin 27 in CD8+ T cell functions and generation of cytotoxic T lymphocytes. J. Biomed. Biotechnol. 2010; 605483.
- Salcedo R, Stauffer JK, Lincoln E, Back TC, Hixon JA, Hahn C, et al. IL-27 mediates complete regression of orthotopic primary and metastatic murine neuroblastoma tumors: role for CD8+ T cells. J Immunol. 2004; 173: 7170- 7182.
- Chiyo M, Shimozato O, Iizasa T, Fujisawa T, Tagawa M. Antitumor effects produced by transduction of dendritic cells-derived heterodimeric cytokine genes in murine colon carcinoma cells. Anticancer Res. 2004; 24: 3763- 3767.
- Shimizu M, Shimamura M, Owaki T, Asakawa M, Fujita K, Kudo M, et al. Antiangiogenic and antitumor activities of IL- 27. J Immunol. 2006; 176: 7317- 7324.
- Oniki S, Nagai H, Horikawa T, Furukawa J, Belladonna ML, Yoshimoto T, et al. Interleukin-23 and interleukin-27 exert quite different antitumor and vaccine effects on poorly immunogenic melanoma. Cancer Res. 2006; 66: 6395-6404.
- Yoshimoto T, Morishima N, Mizoguchi I, Shimizu M, Nagai H, Oniki S, et al. Antiproliferative activity of IL-27 on melanoma. J Immunol. 2008; 180: 6527- 6535.
- Matsui M, Kishida T, Nakano H, Yoshimoto K, Shin-Ya M, Shimada T, et al. Interleukin-27 activates natural killer cells and suppresses NK-resistant head and neck squamous cell carcinoma through inducing antibody-dependent cellular cytotoxicity. Cancer Res. 2009; 69: 2523-2530.
- Ho MY, Leu SJ, Sun GH, Tao MH, Tang SJ, Sun KH. IL-27 directly restrains lung tumorigenicity by suppressing cyclooxygenase-2-mediated activities. J Immunol. 2009; 183: 6217- 6226.
- Cocco C, Giuliani N, Di Carlo E, Ognio E, Storti P, Abeltino M, et al. Interleukin-27 acts as multifunctional antitumor agent in multiple myeloma. Clin Cancer Res. 2010; 16: 4188-4197.
- Kastelein RA, Hunter CA, Cua DJ. Discovery and biology of IL-23 and IL- 27: Related but functionally distinct regulators of inflammation. Annu Rev Immunol. 2007; 25: 221–242.
- Awasthi A, Carrier Y, Peron JP, Bettelli E, Kamanaka M, Flavell RA, et al. A dominant function for interleukin 27 in generating interleukin 10-producing anti-inflammatory T cells. Nat Immunol. 2007; 8: 1380–1389.
- Fitzgerald DC, Zhang GX, El-Behi M, Fonseca-Kelly Z, Li H, Yu S, et al. Suppression of autoimmune inflammation of the central nervous system by interleukin 10 secreted by interleukin 27-stimulated T cells. Nat Immunol. 2007; 8: 1372–1379.
- Stumhofer JS, Silver JS, Laurence A, Porrett PM, Harris TH, Turka LA, et al. Interleukins 27 and 6 induce STAT3-mediated T cell production of interleukin 10. Nat Immunol. 2007: 8: 1363–1371.
- Hall AO, Beiting DP, Tato C, John B, Oldenhove G, Lombana CG, et al. The cytokines interleukin 27 and interferon-gamma promote distinct Treg cell populations required to limit infection- induced pathology. Immunity. 2012; 37: 511–523.
- Karakhanova S, Bedke T, Enk AH, Mahnke K. IL-27 renders DC immunosuppressive by induction of B7-H1. J Leukoc Biol. 2011; 89: 837–845.
- Barrera L, Montes-Servín E, Barrera A, Ramírez-Tirado LA, Salinas-Parra F, Baales-Méndez JL, et al. Cytokine profile determined by data-mining analysis set into clusters of non-small-cell lung cancer patients according to prognosis. Annals of Oncology. 2015; 26: 428–435.
- Duan M, Ning Z, Fu Z, Zhang J, Liu G, Wei Q, et al. Decreased IL-27 Negatively Correlated with Th17 Cells in Non-Small-Cell Lung Cancer Patients. Mediators of Inflammation. 2015; 802939.
- Li S, You WJ, Zhang JC, Zhou Q, Shi HZ. Immune Regulation of Interleukin-27 in Malignant Pleural Effusion. Chinese Medical Journal. 2015; 128: 1932- 1941.
- Pantic Bisevac J, Stanojevic I, Mijuskovic Z, Banovic T, Djukic M, Vojvodic D. High interleukin 27 production is associated with early clinical stage and localized disease in patients with melanoma. J Med Biochem. 2016; 35: 1–8.
- Kachroo P, Lee MH, Zhang L, Baratelli F, Lee G, Srivastava MK, et al. IL-27 inhibits epithelial-mesenchymal transition and angiogenic factor production in a STAT1-dominant pathway in human non-small cell lung cancer. Journal of Experimental & Clinical Cancer Research. 2013; 32: 97.
- Lee MH, Kachroo P, Pagano PC, Yanagawa J, Wang G, Walser TC, et al. Combination Treatment with Apricoxib and IL-27 Enhances Inhibition of Epithelial-Mesenchymal Transition in Human Lung Cancer Cells through a STAT1 Dominant Pathway. J Cancer Sci Ther. 2014; 6: 468-477.
- Airoldi I, Tupone MG, Esposito S, Russo MV, Barbarito G, Cipollone G, et al. Interleukin-27 re-educates intratumoral myeloid cells and down-regulates stemness genes in non-small cell lung cancer. Oncotarget. 2015; 6: 3694- 3708.
- Naumnik W, Naumnik B, Niewiarowska K, Ossolinska M, Chyczewska E. Novel cytokines: IL-27, IL-29, IL-31 and IL-33. Can they be useful in clinical practice at the time diagnosis of lung cancer? Exp Oncol. 2012; 34: 348-353.
Citation: Karlicic V, Vukovic J, Stanojevic I, Sotirovic J, Peric A, Jovic M, et al. IL-27 Concentration in Systemic Circulation and Tumor Micro-Circulation Samples of Sclc and Nsclc Patients; Association with Tumor Size, Histological Type and Presence of Metastases. Austin J Clin Immunol. 2016; 3(1):1030. ISSN : 2381-9138