
Research Article
Austin J Clin Immunol. 2021; 7(1):1041.
An Alternative to Process Integration: Clarification and First Step of Monoclonal Antibodies Purification Using Flocculation, Adsorption and Filtration
Carvalho RJ*
Federal University of Rio de Janeiro (UFRJ), Chemical Engineering Program, COPPE, Brazil
*Corresponding author: Rimenys J Carvalho, Federal Rural University of Rio de Janeiro (UFRRJ), Chemical Engineering Department, BR 465, Km 7, Seropédica/RJ 23897-000, Brazil
Received: March 06, 2021; Accepted: March 27, 2021; Published: April 03, 2021
Abstract
Background: New strategies for up and downstream integration process are being required for end-to-end continuous process for recombinant protein. Single-use materials as well as low cost and easy processing are very welcome for this development. Monoclonal antibodies are largely produced in biopharmaceutical industry what makes it a good protein model to be used in this process.
Objective: A new strategy for integrating up and downstream processes for monoclonal antibodies was developed as a three-step process: flocculation of the cell culture harvest (batch mode); followed by anion-exchange chromatography for impurities adsorption in a slurry, and single-pass tangential flow filtration (both semi-continuous mode).
Methodology: To develop this integrated process, separated studies of flocculation and adsorptions conditions with anion-exchange beads were performed. After defining the optimal conditions, flocculation was performed, and the supernatant was pumped in a vessel along with beads suspension for adsorption. The adsorption was carried out in a residence time determined in the previous studies. Then, the suspension with supernatant was filtered where the antibodies were recovered in the permeate.
Results: Under the adsorption conditions applied (pH 6.5 and 250 mM NaCl), the purer antibodies were recovered in the permeate, whereas the beads with adsorbed impurities remained in the retentate. Steady state profile was obtained during adsorption and filtration for all conditions studied, where no loss of product was obtained. Differently, when the overall process was considered, global yields varied between 61% and 90% due to the void volumes of the runs. Additionally, higher concentration of beads (sample/beads ratio of 41) enabled high amount of impurities removal: 98.9% of DNA and approximately 70% of host cell proteins. Regarding the retention devices studied, depth filter yielded lower void volumes when compared to lamella settler (higher than 5-fold), begetting a global antibodies recovery of 90.4% in 20% higher productivity.
Conclusion: Combining both clarification and impurities removal protocols into a single one proved to be a simple, efficient and fast alternative, which improvement could be obtained by its fully automatization.
Keywords: Anion exchange chromatography; Flocculation; Integration process; Monoclonal antibodies; Protein purification; Single-pass tangential flow filtration; Semi-continuous process
Introduction
Currently, Monoclonal Antibodies (mAbs) are the most produced biopharmaceuticals, given their various therapeutic applications, including several types of cancer, autoimmune diseases and arthrosis [1]. Indeed, more than 50% of the biopharmaceuticals approved in the market today are mAbs or mAb-related products – fragments or fusion proteins with partial mAb structure [1,2]. The growing number of mAb derivatives represents an important share in the market, which reached more than US$ 123.03 billion on sales in 2019- an 14% increase is expected through to 2027 [3]. In view of this, mAbs have attracted a great deal of attention from industries and research groups all over the world seeking to improve production processes.
In the last 20 years, significant improvements in upstream mAbs production processes led to high titers of these products, reaching values up to 25g/L [4]. Consequently, the bottleneck of the production shifted to the downstream processes. Indeed, in the last years, several improvements on stationary phases and developments on new processes have emerged in order to overcome this specific downstream issue [5]. Innovation on process development has brought up the new paradigm of “continuous process” (both up and downstream), notion which has attracted the attention of traditional biopharmaceutical producers due to advantages such as: higher productivity, higher flexibility, lower footprint, lower work volume and higher automatization of the process [6,7].
The so-called continuous upstream process, which has first been applied to unstable proteins, had its mode of operation extended to more stable proteins such as mAbs, due to several advantages entailed by this process [7]. In contrast, continuous downstream processes had a late development because of their complexity (resulting from the several purification steps required in it) [5,7]. Most of the continuous downstream methods currently developed comprise one purification step only. Periodic Countercurrent Chromatography (PCC) [8] and Countercurrent Tangential Chromatography (CCTC) [9], for instance, illustrate one-step-continuous-purification processes. Yet, both allow for combination or integration into other processes such as Tangential Flow Filtration (TFF) and negative mode chromatography. Furthermore, combining continuous and periodic processes among themselves is equally possible [7].
Continuous or not, the production process requires an efficient system to integrate both up and downstream process; to avoid product loss and to clarify the supernatant as much as possible [7,10]. Filtration has successfully replaced centrifugation over the years given its lower cost, highly effective and flexible technique. In addition, several options on single-use products are currently available in the market, and some types of filtration, such as TFF, are easily adapted to continuous or straight-through processes [11-13]. New techniques exploring TFF have emerged, aiming to employ a continuous purification mode that easily integrates with upstream process, e.g. Countercurrent Tangential Chromatography (CCTC) [9,14]. In CCTC-where chromatographic particles flow through sequential hollow fibers-adsorption, washing and elution steps take place just as in an ordinary chromatographic column, with the advantage of no need for packing [7,9]. Thus, clarification and capture purification steps would be performed in a single step. Regardless of the filter’s high efficiency, their capacity might be drastically reduced when large cells concentration is obtained from cell cultures. An alternative to this problem consists in the prior flocculation of the cell culture, whereby the presence of particles is largely reduced given the fast sedimentation of the formed flocs-strategy which has been extensively demonstrated in the literature [15,16].
Indeed, several studies can be found in the literature on mammalian cells’ flocculation at the clarification stage by using different flocculating agents, such as the cationic polymers PDADMAC and chitosan [17,18], caprylic acid [19], and calcium phosphate [20]. Flocculation presents clear advantages besides being a low cost and efficient method: it not only removes cells and debris but also impurities such as host cell DNA, Host Cell Proteins (HCP) and viruses [18,21,22].
In this work, a new process to integrate clarification with first purification step for mAbs was developed by combining flocculation, anion exchange chromatography in a slurry, and single-pass tangential flow filtration techniques; all performed in a sequential process in which the first part (flocculation) is performed in a batch mode, whereas the second one (adsorption and tangential flow filtration) is as a semi-continuous mode. In this proof-of-concept work, a steady state profile was attempted during adsorption and filtration steps of the system.
Materials and Methods
Materials
The cells used for production were CHO (Chinese Hamster Ovary Cells) DP12, producing humanized IgG1 anti-IL8 monoclonal antibodies. The base medium TC-LECC and the feed medium TCX7D used were both from Xell (Germany). With regard to the salts used, PBS tablet, sodium chloride, sodium phosphate dibasic, potassium phosphate monobasic and sodium hydroxide were purchased from Sigma-Aldrich (USA). Q-Sepharose™ Fast Flow was purchased from GE Healthcare (USA). All the ultrapure water used was purified by the system Milli-Q from Merck Milllipore (USA). Polydiallyldimethylammonium Chloride (PDADMAC) was purchased from Merck (USA), and chitosan was purchased from Sigma (USA).
The tangential filtration system applied was QuixStand™ benchtop system from GE Healthcare (Sweden) with a hollow fiber CFP-2-G-4X2MA from GE Healthcare (USA) of 0.2μm cut-off, 60cm high, featuring 650cm2 of membrane surface area, 1.75mm lumen, supported pressure in the feed of 25psig, and Transmembrane Pressure (TMP) of 15psig. The transmembrane pressure was obtained through pressures gauges measurements of this system.
The High-Performance Liquid Chromatography (HPLC) system, used for analytical purposes, was Shimadzu Prominence (USA), equipped with three pumps, column oven, automatic injector with temperature control, and UV 280nm detector.
Methodology
Cells production: CHO Productions were performed using Erlenmeyer flasks at 37ºC and 180rpm and incubated in a 5% CO2 environment using base medium TC-LECC. The cultures were usually harvested in the 5th day after inoculation. In order to reach higher cell densities, a manual feeding to the flask was generated daily, based on cell exponential growth, using the concentrated feed medium TCX7D. Cells counting and viability were determined by the trypan blue method using Vi-Cell XR automated counter from Beckman Coulter (USA).
Flocculation studies: Previous flocculation studies have been carried out with the use of two polymers as flocculation agents, namely PDADMAC and chitosan [18]. In this study, protocols were applied by considering the best conditions observed in a previous study: 5pg/ TC chitosan flocculating agent applied to the suspension at pH 6.5 (cell broth plus flocculation agents), then further agitated at 100rpm at room temperature (approximately 25ºC) for 30min. Finally, once the agitation was over, the resulting flocs were let to settle down prior to pumping the supernatant into the system.
Anion exchange adsorption studies: The Anion Exchange (AEX) adsorption studies were carried out using Q-Sepharose™ Fast Flow as adsorption beads in suspension. The kinetics studies were performed in 1.5mL tubes using a ratio of 1:10 particles: sample volume. Agitation was performed at 1200rpm using a Thermo Mixer from Eppendorf (USA) at room temperature.
Samples from Flocculated Supernatant (FSN) were taken after 0, 0.5, 1, 2, 5, 10, 30 and 60 min of adsorption.
Moreover, adsorption studies were performed at pH 6.5, under different NaCl concentrations of 50, 150, 250 and 350 mM, by using a flocculated supernatant diluted twice in Phosphate Buffered Saline (PBS) with 10mM phosphate + 150mM NaCl plus the supplementation with NaCl. In all adsorption studies, the particles were first equilibrated with their respective buffer three times at 1200rpm at room temperature. The ratio of particles to buffer was the same one applied to the samples.
With regard to sample preparation, the supplementation of PBS with NaCl was doubled in concentration. The agitation and temperature conditions were the same as in previous studies.
Sequential clarification and AEX adsorption system: The sequential process was performed in three different steps further to cell cultivation: (a) flocculation for cell separation and impurities removal (batch mode), (b) AEX adsorption in a slurry as a first purification step, and (c) single-pass Tangential Flow Filtration (TFF) for clarification (semi-continuous mode – b and c), (Figure 1).
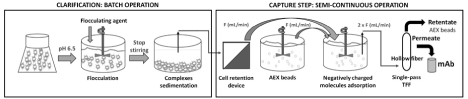
Figure 1: Scheme representing the clarification and negative adsorption integration system: upon the adjustment of a cell culture production to pH 6.5, flocculation
was started by means of chitosan. Subsequently, the complex was left to sediment once stirring has stopped. The supernatant was then placed into another
flask to be adsorbed upon exposure to Q Sepharose beads (AEX beads). Both supernatant and beads were pumped in the same Flow rate (F). The supernatant
could pass through a cell retention device before being added in the adsorption flask. After the residence time, the impurities adsorption was completed, and the
suspension was pumped into the hollow fiber. The purified mAb was recovered in the permeate and the beads were recovered in the retentate. These were sent
later for Cleaning-In-Place (CIP) and sanitization for further use.
The flocculation and AEX adsorption conditions had been previously studied with the same starting material used in the process performance, as described above.
As represented in Figure 1, monoclonal antibodies were produced in a flask using CHO DP12 cells, after which cells were counted. Subsequently, the pH of the cell broth was reduced to 6.5; chitosan was added into the flask at a final concentration of 5pg/TC; the flask was placed under agitation (at 100rpm) for 30min.
After settling down, the supernatant was pumped into a stirred vessel along with Q Sepharose beads suspension at a concentration two-fold higher than the final one planed for the adsorption step (in a stirred flask).
Beads suspension and supernatant were pumped in at the same flow rate to obtain a two-fold diluted supernatant during AEX adsorption. The residence time for adsorption was set after the kinetic adsorption studies. Furthermore, the suspension (supernatant plus AEX beads) was pumped into a hollow fiber membrane of 0.22μm cut-off and manually controlled via Transmembrane Pressure (TMP) through the use of pressure gauges.
At this AEX adsorption condition, mAbs did not interact with the beads since its Isoelectric Point (pI) was around 8.0. Hence, the soluble monoclonal antibodies were extract from the permeate, while in contrast the beads with interacted impurities were recovered from the retentate fraction. Afterwards, these beads were recovered for regeneration, cleaning and sanitization before being reused.
Aiming to enhance the process performance, after the flocculation step, trials were performed so as to compare two different cell retention devices, namely: a laboratory-scale lamella settler CS10 from Biotechnology Solutions (USA), and a depth filter Clarisolve from Merck (USA).
Analytical methods:
Mab quantification: The humanized mAb Immunoglobulin G (IgG) anti-IL8 quantification was performed with protein A chromatography using PA ID Sensor Cartridge column from Applied Biosystem (USA), as described elsewhere [23]. The adsorption conditions were set with buffer 50mM phosphate and 150mM NaCl at pH 7.4, whereas the elution was performed with buffer 12mM HCl and 150mM NaCl at pH 2–3. Analyses were performed with a HPLC from Shimadzu (Japan) coupled with SDP-M30A photodiode array detector.
Host cell DNA quantification: Residual DNA was determined with the Quant-iTTM PicoGreen® double-stranded DNA assay kit from Invitrogen (USA). The analysis was performed using a microplate fluorometer Victor™ EnLite™ from Perkin Elmer as in the manufacturer’s protocol.
Total proteins quantification: Total protein concentrations were quantified with the Micro BCA™ protein assay kit from Thermo Fisher Scientific (USA); Bovine Serum Albumin (BSA) was used as a standard. After performing the assay according to the manufacturer’s instructions, samples were read using the microplate reader Power Wave HT with the software KCJunior from Biotek (USA).
Host cell proteins quantification: CHO Host Cell Protein (HCP) concentration was quantified with the CHO HCP ELISA kit from Cygnus Technologies (USA), following the protocol recommended by the manufacturer.
MAbs aggregation quantification: The quantification of aggregated forms was accomplished through size exclusion chromatography using HPLC from Shimadzu (Japan), coupled with SDP-M30A photodiode array detector. The column used was TSKgel G3000SWXL from Tosoh Bioscience (Japan).
The chromatographic process was performed at 1mL/min with PBS at pH 7 as the mobile phase in an isocratic method.
Sodium Dodecyl Sulfate Polyacrylamide Gel Electrophoresis (SDS-PAGE): SDS-PAGE was performed according to Laemmli’s method [24], with polyacrylamide gels at 12% and silver staining according to the standard protocol [25]. Further to staining, the gel was scanned-using the software ImageJ 1.8.0 (USA)-to determine mAb products’ purity.
Results
Setting the sequential clarification and first purification step conditions
In this integrated system, cell settling by flocculation and anion exchange adsorption were applied to adsorb impurities from the cell culture harvest; herein analyzed in terms of DNA and total proteins, which includes host cell proteins (HCP). At the conditions applied in this research, it was not expected to obtain mAb adsorption, whereas pH values used were below the product isoelectric Point (pI) (approximately 8.0).
Previous research on flocculation has been carried out comparing chitosan and PDADMAC as cationic flocculating agents [18]. According to these studies, optimal conditions are met at chitosan 5pg/TC concentration and pH 6.5.
Previous studies on AEX adsorption were adopted as a reference to set the kinetics applied to the samples (supernatant after flocculation at pH 6.5, same adopted in flocculation protocols). These first results showed that after 10 min of equilibrium, the beads reached their maximum capacity for DNA and total proteins adsorption (Figure 2).
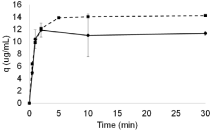
Figure 2: Graphic representing Q Sepharose adsorption kinetics of DNA
(dotted line with squares) and total proteins (straight line with circles) at pH
6.5.
Based on this evidence, two rounds of flocculation followed by adsorption were performed. The studies were performed at the pH values 6.5 and 7.0, so as to determine the influence of pH variations in the whole system. The ratio of AEX beads per sample was set as to favor DNA removal capacity, and therefore does not accounts for shifts in adsorption capacity related to pH fluctuation. The samples used in the adsorption studies presented around 182ng/mL of DNA concentration; considering a Q-Sepharose adsorption capacity of 15μg of DNA per mL (82-fold higher than the DNA in the sample) registered for the samples used in this work (Figure 2), a ratio of 1 volume of bead to 82 volumes of sample was first applied.
As described in Table 1, the lowest pH value entailed the highest mAb recovery and DNA removal rates. In contrast, although a pH value 7 increased total proteins removal by almost 5- fold, these included mAbs.
Steps
mAb Recovery (%)
DNA Removal (%)
Total Proteins Removal (%)
pH
6.5
7
6.5
7
6.5
7
Cell culture broth
100
100
0
0
0
0
Flocculation
100±23.9
75.7±3.9
94.4±0.7
67.2±2.1
2.4±1.4
22.1±1.5
AEX
69.7±7.7
47.2±3.3
99.8±0.8
87.5±0.5
8.9±2.6
41.8±1.3
Table 1: Results from sequential purification with flocculation followed by Anion Exchange Adsorption (AEX) using Q-Sepharose beads at pH values 6.5 and 7.0 using PBS N=2.
Thus, a pH value of 6.5 was adopted for the next studies, even though the associated mAb recovery rate of 70% could still be improved. In this way, adsorption studies were carried out at different conductivities-by changing the concentration of NaCl-in order to mitigate mAb’s adsorption. The results can be observed in Figure 3.
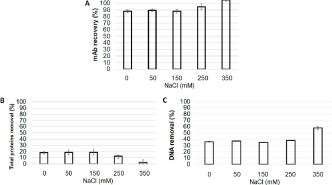
Figure 3: MAb recovery (A), total proteins removal (B) and DNA removal
(C) from flocculated supernatant obtained with CHO cultivation subsequently
adsorbed by Q Sepharose beads at 0, 50, 150, 250 and 350 mM NaCl in a
PBS buffer at pH 6.5.
The first studies used PBS (10mM phosphate supplemented with 150mM NaCl) to prepare the beads suspension prior to the addition into the supernatant. Given the two times dilution of the samples and suspension beads, the final concentration of NaCl was 75mM. Conductivities studies were performed with NaCl final concentration in the sample up to 350mM (Figure 3). According to these results, the presence of NaCl mitigated the proteins adsorption, but not the DNA’s. The presence of 350mM NaCl reduced the proteins adsorption to close to 0%. On the other hand, the mAb was totally recovered at this concentration.
The fact that no proteins were adsorbed suggests no competition with DNA interaction. Thus, an increase on DNA removal was observed, since NaCl concentration was not high enough to mitigate the DNA adsorption on the Q Sepharose.
At 250mM NaCl mAb adsorption was mitigated, resulting in a recovery rate of around 95%. However, it was still possible to remove proteins, since more than 10% of the total proteins were cleaned from the sample, and 40% of DNA was removed. Based on this, the concentration of 250mM NaCl set in the AEX adsorption was identified as for its potential to advance further studies on systems integration.
Running the system: clarification and first purification step integration and results
In order to evaluate and prove the concept of the process, six runs were performed applying distinct conditions (Table 2). Among these runs, there were some variations regarding cell density and viability, the use of different particles retention device and the ratio of Q Sepharose to sample volumes.
Runs
Total cells (106cel/mL)
Viability (%)
MAb production (mg/L)
Device for particles retention
Ratio Sample/AEX
Feed* vol. (mL)
Recovered vol. (mL)
Retentate vol. (mL)
Void vol.** (mL)
1
8.3
87.6
206
None
82
500
850
100
50 (F)
2
12.5
95.2
140
Settler
82
384
525
60
183 (S)
3
17
88.8
257
Settler
82
480
594
80
286 (S)
4
24
80.4
357
Settler
41
512
672
110
242 (S)
5
13.2
91.9
152
Filter
41
450
770
98
32 (DF)
6
9.5
87.7
192
Filter
41
495
840
120
30 (DF)
*Process volume: Recovered + retentate + void = 2x feed
**F is a void volume from sedimented flocs, S from the settler void volume, and DF from the depth filter void volume.
Table 2: Process conditions of the starting material (total cells and their viability), presence and type of retention device, ratio of samples to AEX beads volume, and volumes of feed, recovery, retentate and void.
After running the first condition, the beads were recovered in the retentate along with particles such as flocs, cells and cell debris, given that no particles retention device was used after flocculation. The presence of these impurities made the recovery of Q Sepharose beads difficult, even after waiting for their sedimentation and avoiding their pumping what generated a void volume of sample (50mL).
To overcome these issues, a particles retention device was employed after the flocculation step. Indeed, it allowed pumping the supernatant straight away after flocculation and successfully retained the particles. In addition, the beads were recovered without any particles by the end of the process, facilitating their regeneration, cleaning and sanitization. Lamella settler and depth filter were used as particles retention devices in these studies.
The settler was efficient at retaining particles (Table 3, runs 2, 3 and 4), however two disadvantages came along with the use of this device: the large void volume and the limited flow rate options available in the system. For the size of the settler used in this work (CS10), the maximum flow rate (Figure 1) observed was 6.4mL/ min. According to the manufacturer, higher flow rates would reduce settler’s efficiency.
Runs
MAb recovery (%)
DNA removal (%)
HCP removal (%)
Mab relative purity (%)*
Mab productivity (mg/L.h-1)
1
85.4
93.8
20.7
58.3
84
2
78.6
85
32.4
66.7
119.4
3
63.2
96.6
52.8
62.4
156.7
4
60.9
92
39.4
65.9
127.8
5
90.1
98,8
52.9
62.8
174.1
6
90.4
98,9
69.8
66.1
196
*Calculated by densitometry of SDS-PAGE.
Table 3: Global results of IgG recovery, host cell DNA and proteins removal, and productivity of the six runs.
This device also provided large void volumes (between 120 and 176 mL), which reduces the product volume recovered after purification (68%, 62% and 66% of the runs 2, 3 and 4, respectively). Consequently, lower mAb recoveries were detected, reaching the minimum value of 60.9% in run number 4 (Table 3).
The depth filter was also able to retain all particles, by exerting a flow rate 80% higher than the settler (F=11.5mL/min). This device provided lower void volumes when compared to the settler-of around 30mL in both runs-and therefore, higher volume was recovered after purification (86% and 85% in runs 5 and 6, respectively), increasing the mAb recovery up to 90%. The depth filter runs provided higher recovery rates when compared to the ones performed with the settler and to run 1 (without cell retention device). Although the AEX beads recovery was difficult to perform in the run 1, the final mAb recovery value was comparable to the one detected for runs 5 and 6 (85.4%), and higher than the one obtained with the settler device (given the lower void volume obtained from the settled flocs) (Table 2). The first purification step for mAbs stablished in the industry, protein A chromatography, attains more than 95% mAb recovery of efficiency. However, this method is highly expensive and protein A leaching in the samples is reported [26]. This integrated system reaches 90% of recovery, which is lower than protein A, but much higher than alternative mAbs purification, such as activated carbon with values around 60% [27].
With regard to the removal of impurities, all runs yielded a DNA removal higher than 90%, except for run number 2 (85% removal). Overall, the run number 4 presented the lowest DNA removal ratio, regardless of the exposure to a higher concentration of adsorption beads (Table 3). This result could be explained by the lower viability of the cells used as the starting material, which increased both DNA and HCP amounts in the sample. In order to run an adsorption as performant as the ones at the other conditions, a concentration of beads higher than the one used in this study could be required.
In general, higher HCP removals rates were observed when the ratio of sample to AEX beads was at 41. An exception was observed in the run number 3, which showed a higher removal rate than in the run number 4 (Table 3). This result could be explained by the higher cells viability (lower presence of HCP) and lower volume recovered, which resulted in higher loss of the total proteins.
To corroborate this with other results, the runs number 5 and 6 presented the best productivities, 174.1 and 196 mg/L.h-1, respectively (Table 3). These higher values could be explained by their shorter processing time, in turn resulting from an 80% higher flow rate. In addition to the speed of the process, the amount of mAb produced also affected the productivity (Table 2). For instance, the run number 4 resulted in a higher cell concentration as well as a higher mAb production (357mg/L) (which in turn could have favored its final productivity); yet, it is worth mentioning that this was the longest among the six runs. Thus, lower productivity than runs 3, 5 and 6 was detected in this run regardless of the higher mAb production. Regarding mAb purity, this system cleaned up the supernatant by densitometry and yielded from 55% up to 65% of relative purity based on proteins despite the system’s focus on DNA removal (Table 3). Partial removal of proteins is observed in the SDS-PAGE through samples from runs 5 and 6 (Figure 4). The run number 1 presented the worst result among the series, what might have been due to the presence of cells/cells debris during adsorption.
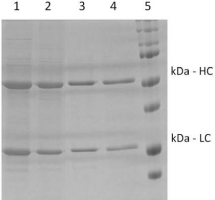
Figure 4: SDS-PAGE of samples from the best runs undergoing the
clarification and purification process developed in this work using – by
combining flocculation, AEX adsorption and single-pass TFF. Legend: 1)
Supernatant from the cell culture of run number 5; 2) Supernatant after
flocculation from run number 5; 3) Sample from permeate fraction of run
number 5; 4) Sample from permeate fraction of run number 6; 5) Molecular
weight marker revealing the Heavy Chain (HC) at 50kDa and the Light Chain
(LC) at 25kDa.
The presence of mAb aggregates was also analyzed in the harvest, after the flocculation, and in the permeate further to AEX adsorption and filtration (Table 4). The results showed that the aggregates were formed during the cultivation. The flocculation did not promote any aggregation, except for in the run number 1, where the percentage of aggregates was more than 2-fold higher after flocculation. The process removed aggregates at all conditions, after filtration; a low percentage still remained in the runs number 2, 3 and 4, the latter which had the highest aggregates concentration in the harvest.
mAb Aggregates (%)
Harvest
Flocculation
Permeate
1
0.2
0.5
0
2
1.7
1.1
0.5
3
0.8
0.8
0.1
4
2.3
2.3
0.1
5
0.7
0.3
0
6
0.7
0.1
0
Table 4: SDS-PAGE of samples from the best runs undergoing the clarification and purification process developed in this work using – by combining flocculation, AEX adsorption and single-pass TFF. Legend: 1) Supernatant from the cell culture of run number 5; 2) Supernatant after flocculation from run number 5; 3) Sample from permeate fraction of run number 5; 4) Sample from permeate fraction of run number 6; 5) Molecular weight marker revealing the Heavy Chain (HC) at 50kDa and the Light Chain (LC) at 25kDa.
Analysis of the steady-state part of the process
According to Figure 1, the process consists in a semi-continuous mode of operation after a flocculation step. Due to this characteristic, a steady state profile could be reached, as shown in graphics A, B and C of Figure 5 (which depicts the analysis of mAb, DNA and total proteins concentrations over time).
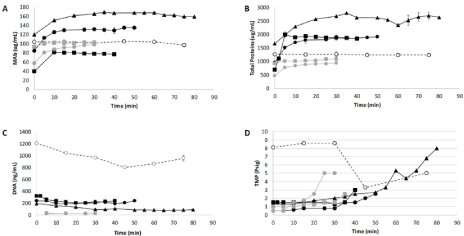
Figure 5: Graphics representing mAb concentration (A), total proteins concentration (B), host cell DNA concentration (C) and transmembrane pressure – TMP
(D) over time, obtained after tangential flow filtration (concentrations). Run 1: Black open circle and dashed line; Run 2: Black squares; Run 3: Black circle; Run 4:
Black triangle; Run 5: Grey square and Run 6: Grey circle.
The fluctuations observed in the concentrations of mAb, DNA and total proteins stem from differences with regard to cell cultivation, i.e., the higher the cell density, the higher the mAb production, whereas the lower the viability, the higher presence of the contaminants host cell DNA and proteins. This can be verified by the results from runs number 5 and 6: not only they were submitted to similar conditions, but also their analytes concentrations were comparable.
Indeed, other conditions might have played a role as well. For instance, in runs number 2 and 5 similar cell densities and viability values were observed, although differences concerning beads concentration during the AEX adsorption caused unequal results on impurities adsorption. As observed in run number 2, the lower presence of beads (black squares in Figure 5) resulted in a higher concentration of DNA and total proteins during the steady state, when compared to run number 5 (grey squares in Figure 5).
The pressure exerted by the TFF was manually monitored (Figure 5D). The importance of this monitoring lies in maintaining the stability of the system by keeping the working flux lower than the critical one [28]. Herein, a low working flux was set in the TFF system, and further the percentage of particles pumped into the membrane was compared to literature data; specifically, countercurrent tangential chromatography was adopted as a reference process given its similarity [9,14]. In these authors’ work, beads were used in a slurry and pumped into hollow fibers to purify proteins using 115L/m2/h (LMH) of permeate flux. Herein, the flux operated was set around 11 LMH in runs number 1 to 4, and around 23 LMH in runs 5 and 6. The permeate fluxes were limited due to the lower TFF feeding flow rate provoked by the retention devices settler and depth filter. A TFF feeding flow rate of 12.8mL/min, lower than compared to the literature (51mL/min) [14], was applied to run 1 as well, even though the TMP pressure was higher than in other conditions (Figure 5D – open circle). This was probably caused by the presence of flocs and cell debris along with the Q Sepharose beads. The runs number 2 and 3 presented the more stable TMP along the runs, when compared to runs number 4, 5 and 6. This, in turn, was probably due to the lower amount of the particles detected in the suspensions. None of the six runs has reached the maximum pressure of the hollow fiber (15psig); in fact, a manual control of retentate outlet flow was necessary to control the TMP pressure.
Moreover, Table 5 presents the results on the efficiency of mAb purification based on mAb recovery and purification factors related to HCP and DNA during the steady state profile of the runs. The values were calculated from measurements 5 min further to the permeate fractions collection.
Runs
MAb recovery at steady state (%)
Purification factors at steady state*
HCP
DNA
1
49.2 ± 0.3
1.1
4.3
2
48.8 ± 0.1
1.2
14.9
3
50.1 ± 1.2
1.5
17.8
4
46.0 ± 0.6
1.1
34
5
49.4 ± 0.5
1.9
73.7
6
50.7 ± 2.3
3
85.7
*Deviation values up to 5% of the analytical methods used (ELISA for HCP and PicoGreen for DNA).
Table 5: Percentage of mAb recovery and purification factors of HCP and DNA at the steady state phase of the runs. Values calculated from permeate fractions.
The recovery of all processes was estimated in 50% or close, denoting no loss of mAb during the process since the supernatant was diluted twice during the AEX adsorption. On the other hand, the purification factor demonstrated larger differences depending on the process conditions. As expected, runs number 4, 5 and 6 presented higher DNA purification factors when higher concentrations of Q Sepharose beads were used. A similar profile was obtained with HCP, but only at the condition where a depth filter was integrated into the system (runs 5 and 6).
The lower purification factor detected in run number 4 might not be due to the cell retention device functioning, but, instead, to the low viability. As discussed above, higher beads concentration values (in comparison to those observed in this work) could have enhanced the removal of impurities; since a higher amount of HCP was present in the harvest: approximately 330μg/mL in run 4, and 150μg/mL in runs 5 and 6.
The worst scenario regarding purification factors was observed in run number 1. Despite the lower beads’ concentration, the presence of flocs, cells and cells debris during the AEX adsorption could probably disturb the interaction between beads and impurities; since DNA purification values were more than 3-fold lower in comparison to other conditions with the same concentration of AEX beads.
Discussion
In this work, a new process combining clarification with a first purification step for mAb was developed aiming to integrate up and downstream processes. More specifically, flocculation, followed by AEX adsorption, and single-pass TFF were the techniques composing such process. The first results suggest an efficient and fast process, not only able to clarify cell culture harvest-by means of a low cost and simple procedure such as flocculation-but also to remove more than 95% of host cell DNA and more than 50% of HCP from the cell culture supernatant.
The use of a particles retention device after flocculation improved the system, and the adsorption beads used in the process could be recovered for reuse. For instance, the use of such devices avoided the waiting time for sedimentation and enhanced the productivity. With regard to the average productivity, the process with a depth filter showed to be more efficient (average productivity of 185mg/L.h-1 in runs number 5 and 6) when compared to the one using a settler (127.8mg/L.h-1 productivity in run number 4).
This system can easily be operated; however, it provokes product dilution and can be influenced by cell viability. Although dilution occurs when the product concentration is under control at the purification step, the integrated system outlined in this work yields a cleaner supernatant, with less organic load, which could improve the service lifespan of the following purification column. The influence of cell viability in the process efficiency (the lower the viability, the higher the presence of impurities) has already been described in the literature, for purification processes based on non-specific separation principles such as organic load [29]. For instance, a harvest from a cell culture with lower viability (around 80%) was used in run number 4. The adsorption step was performed with a high concentration of AEX adsorption beads (ratio sample: AEX of 41); yet, a low rate of HCP and DNA removal was observed in run number 4, which points to the need of a higher concentration of AEX beads in order to increase impurities adsorption.
Although all runs were carried out manually, automatization could enhance both the process monitoring and the control over variables such as flow rate and pressure. In addition, automatization could make it possible to design a process directly from the flocculation step, in which the flocculants would be pumped along with the harvest, at a pre-defined concentration, into another flask. Both harvest and agent would be kept at agitation during the residence time required to flocculate, prior to pumping to the following step. Even though the manual control of a partially semi-continuous process assured a steady state profile-as it can be deduced from mAb, DNA and total proteins concentrations (Figure 5) an improvement of the process can be obtained through its automatization, resulting in a straight through process which could enhance the integration of up and downstream.
Conclusion
In conclusion, in this proof-of-concept the integration of clarification with the first purification step has proved to be efficient at purifying mAbs. Indeed, it consists in a flexible platform which enables to operate the adsorption beads at different conditions, and also to include additional adsorption steps after the first single-step TFF. Alternatively, the system could also be adapted into a continuous operation mode featuring continuous addition of flocculants, although in this case automatization is strongly recommended.
Acknowledgment
The author would like to acknowledge Prof. Leda R. Castilho and Cell Culture Engineering Lab at COPPE/UFRJ for providing workspace to develop this work.
Funding
This study was financed by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES) - Finance Code 001, FAPERJ under the process number E-26/202.188/2015, and by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) through Jovens Talentos edition under the process number 407338/2013-6.
References
- Kaplon H, Reichert JM. Antibodies to watch in 2018. MAbs. 2018; 10: 183- 203.
- U.S. Food and Drug Administration. 2020.
- Monoclonal Antibody Therapy Market Size, Share & Industry Analysis. Fortune Bus Insights 2020.
- Shukla AA, Wolfe LS, Mostafa SS, Norman C. Evolving trends in mAb production processes. Bioeng Transl Med. 2017; 2: 58-69.
- Tripathi NK, Shrivastava A. Recent developments in bioprocessing of recombinant proteins: expression hosts and process development. Front Bioeng Biotechnol. 2019; 7: 420.
- Konstantinov KB, Cooney CL. White paper on continuous bioprocessing. J Pharm Sci. 2015; 104: 813-820.
- Carvalho RJ, Castilho LR. Tools enabling continuous and integrated upstream and downstream processes in the manufacturing of biologicals. In: Continuous biomanufacturing: innovative technologies and methods, Subramanian G., Ed., Wiley-VCH. 2018: 31-68.
- Godawat R, Brower K, Jain S, Konstantinov K, Riske F, Warikoo V. Periodic counter-current chromatography - design and operational considerations for integrated and continuous purification of proteins. Biotechnol J. 2012; 7: 1496-1508.
- Dutta AK, Tran T, Napadensky B, Teella A, Brookhart G, Ropp PA, et al. Purification of monoclonal antibodies from clarified cell culture fluid using protein A capture continuous countercurrent tangential chromatography. J Biotechnol. 2015; 213: 54-64.
- Fan X, Liang Y, Li F, Yu J, Song H, Feng D, et al. Integrated purification of a nanobody using ammonium sulfate precipitation and Capto MMC. J Chem Technol Biotechnol. 2020; 95: 246-254.
- Lindskog E, Clincke MF, Mölleryd C, Chotteau V, Zhang Y, Walsh K. Very high density of CHO cells in perfusion by ATF or TFF in WAVE bioreactorTM. Part I. Effect of the cell density on the process. Biotechnol Prog. 2013; 29: 754-767.
- Dizon-Maspat J, Bourret J, D’Agostini A, Li F. Single pass tangential flow filtration to debottleneck downstream processing for therapeutic antibody production. Biotechnol Bioeng. 2012; 109: 962-970.
- Alex B, Sanaa E, John P, Matthew W. Leveraging single‐pass tangential flow filtration to enable decoupling of upstream and downstream monoclonal antibody processing. Biotechnol Prog. 2018; 34: 405-411.
- Shinkazh O, Kanani D, Barth M, Long M, Hussain D, Zydney AL. Countercurrent tangential chromatography for large-scale protein purification. Biotechnol Bioeng. 2011; 108: 582-591.
- Buyel JF, Fischer R. Flocculation increases the efficacy of depth filtration during the downstream processing of recombinant pharmaceutical proteins produced in tobacco. Plant Biotechnol J. 2014; 12: 240-252.
- Lee JC, Kim DY, Oh DJ, Chang HN. Long-term operation of Depth Filter Perfusion Systems (DFPS) for monoclonal antibody production using recombinant CHO cells: Effect of temperature, pH, and dissolved oxygen. Biotechnol Bioprocess Eng. 2008; 13: 401-409.
- Mcnerney T, Thomas A, Senczuk A, Petty K, Piper R, Carvalho J, et al. PDADMAC flocculation of Chinese hamster ovary cells: Enabling a centrifugeless harvest process for monoclonal antibodies. MAbs. 2015; 7: 413-428.
- Carvalho RJ. Comparison of Cationic Flocculants for the Clarification of CHOderived Monoclonal Antibodies. Biotechnol Bioprocess Eng. 2019; 24: 754- 760.
- Brodsky Y, Zhang C, Yigzaw Y, Vedantham G. Caprylic acid precipitation method for impurity reduction: An alternative to conventional chromatography for monoclonal antibody purification. Biotechnol Bioeng. 2012; 109: 2589- 2598.
- Chen G, Su Z, Li F, Liu HF. Application of calcium phosphate flocculation in high-density cell culture fluid with high product titer of monoclonal antibody. Bioprocess Biosyst Eng. 2017; 40: 703-714.
- Bhalkaran S, Wilson L. Investigation of self-assembly processes for chitosanbased coagulant-flocculant systems: A Mini-Review Int J Mol Sci. 2016; 17: 1662.
- Han B, Carlson JO, Powers SM, Wickramasinghe SR. Enhanced virus removal by flocculation and microfiltration. Biotechnol. Bioprocess Eng. 2002; 7: 6-9.
- dos Santos R, Rosa SASL, Aires-Barros MR, Tover A, Azevedo AM. Phenylboronic acid as a multi-modal ligand for the capture of monoclonal antibodies: Development and optimization of a washing step. J Chromatogr A. 2014; 1355: 115-124.
- Laemmli UK, Beguin F, Gujer-Kellenberger G. A factor preventing the major head protein of bacteriophage T4 from random aggregation. J Mol Biol. 1970; 47: 69-85.
- Ferreira R, Martins-Dias S. Purification of plant complex protein extracts in non-denaturing conditions by in-solution isoelectric focusing. Anal Biochem. 2016; 509: 100-103.
- Shukla AA, Hubbard B, Tressel T, Guhan S, Low D. Downstream processing of monoclonal antibodies-Application of platform approaches. J Chromatogr B Anal Technol Biomed Life Sci. 2007; 848: 28-39.
- Ishihara T, Miyahara M, Yamamoto K. Monoclonal antibody purification using activated carbon as a replacement for Protein A affinity chromatography. J Chromatogr B Anal Technol Biomed. Life Sci. 2018; 1102-1103: 1-7.
- Raghunath B, Bin W, Pattnaik P, Janssens J. Best practices for optimization and scale-up of microfiltration TFF processes. Bioprocess J. 2012; 11: 30-40.
- Carta G, Jungbauer A. Introduction to protein chromatography. Protein Chromatogr. Process Dev. scale-up. Darmstadt: Wiley-VCH. 2010.