
Research Article
Austin J Clin Immunol. 2022; 8(1):1048.
Relationship Between Annual Airborne pollen Levels (1974–2014) and the Occurrence of Idiopathic Dilated Cardiomyopathy, Myasthenia Gravis, Polymyositis/ Dermatomyositis, and Vasculitis Syndrome Based on the National Registry Database of Specific Intractable Disease in Japan: A Retrospective Study
Akira Awaya1,2*, Yoshiyuki Kuroiwa3,4,5
1Dermatology & Epidemiology Research Institute (DERI), 4978 Totsuka-cho, Totsuka-ku, Yokohama, Kanagawa, Japan
2Department of Genome System Science, Yokohama City University, Seto 22-2, Kanazawa-ku, Yokohama, Kanagawa, Japan
3Department of Neurology, University Hospital Mizonokuchi, Teikyo University School of Medicine, 5-1-1, Futago, Takatsu-ku, Kawasaki, Kanagawa, Japan
4Department of Medical Office, Ministry of Finance, 3-1-1, Kasumigaseki, Chiyoda-ku, Tokyo, Japan
5Yokohama City University Graduate School of Medicine, 3-9, Fukuura, Kanazawa-ku, Yokohama, Japan
*Corresponding author: Akira Awaya, Dermatology & Epidemiology Research Institute (DERI), Totsuka-cho, Totsuka-ku, Yokohama 244-0003, Japan
Received: June 23, 2022; Accepted: June 28, 2022; Published: June 30, 2022
Abstract
Background: In Japan, pollen counts increased between 1977 and 1987, including three peaks (1978-1980, 1982, 1984-1986) coinciding with Kawasaki disease (KD) outbreaks. KD and related diseases may be related to pollen exposure (PE).
Methods and Results: To elucidate the effects of PE on outbreaks of intractable muscular diseases and vasculitis syndromes, we evaluated the annual occurrence of disorders in relation to pollen counts using data from a national database. Specifically, we evaluated the occurrence of idiopathic dilated cardiomyopathy (IDCM), myasthenia gravis (MG), polymyositis/dermatomyositis (PM/DM), Takayasu arteritis (TAK), granulomatosis with polyangiitis (GPA), and periarteritis nodosa (PAN). While we did not observe increased disease rates during the first pollen count peak (1978-1980), increased rates of all evaluated diseases were observed during the 1982 and 1984-86 peaks. Furthermore, simultaneous outbreaks coincided with 10pollen count peaks between 1988 and 2013. We observed significant correlations between the annual number of newly registered patients (nRPs) with IDCM, MG, PM/DM, and PAN and annual pollen levels (PL). Significant correlations were also observed between nRPs and the annual PL measured with a lag of 2 years for IDCM and GPA, 1–4 years for PAN, and 6 years for MG, PM/DM, TAK, GPA, and PAN.
Conclusion: Data suggest that the cumulative effects of PE within 6 years prior to diagnosis might possibly trigger onset of muscular specific intractable diseases.
Keywords: Idiopathic Dilated Cardiomyopathy (IDCM); Myasthenia Gravis (MG); Polymyositis/Dermatomyositis (PM/DM); Muscular Specific Intractable Diseases; Takayasu Arteritis (TAK); Granulomatosis with Polyangiitis (GPA), and Periarteritis Nodosa (PAN); Kawasaki Disease (KD); Pollen Levels (PL); Pollen Exposure (PE); Tokyo Metropolitan; Sagamihara City of Kanagawa Prefecture
Abbreviations
IDM: Idiopathic Dilated Cardiomyopathy; MG: Myasthenia Gravis; PM/DM: Polymyositis/Dermatomyositis; TAK: Takayasu Arteritis; GPA: Granulomatosis with Polyangiitis; PAN: Periarteritis Nodosa; SIDs: Specific Intractable Diseases; KD: Kawasaki Disease; PIDs: Pollen-Induced Diseases; Present RPs: Presently Registered Patients; Newly RPs: Newly Registered Patients; AP: Airborne Pollen; PL: Pollen Levels; PE: Pollen Exposure.
Introduction
Idiopathic dilated cardiomyopathy (IDCM) is one of the most common cardiovascular diseases [1,2]. Both IDCM and two other types of cardiomyopathy (hypertrophic cardiomyopathy and restrictive cardiomyopathy) have been registered as specific intractable diseases (SIDs) in Japan [3]. IDCM can be diagnosed only after differential diagnosis regarding cardiac failures such as various types of cardiomyopathy and myocarditis [4]. There is evidence that infants with an allergic constitution may be more susceptible to Kawasaki disease (KD), a systemic vasculitis related to IDCM [5-10].
Previous studies on KD have evaluated the relationship between development of this disease and weekly, monthly, and annual airborne pollen (AP) exposure [6-10]. During 1977–1987, KD patients in all of Japan, specifically in Tokyo and Kanagawa, showed triphasic outbreaks of KD (1978-79, 1982, 1984-86), with the highest peak in 1982. These outbreaks coincided exactly with triphasic peaks of AP release [6,9,10]. KD may be a pollen-induced delayed-type hypersensitivity disease, and suppressed onset may be observed during influenzaepidemics [6-10].
Successive epidemiological studies have evaluated the occurrence of another vasculitis, Takayasu arteritis (TAK) [11]. Research has shown an increase in TAK occurrence starting in 1984,based on data from national databases for presently registered patients (present RPs), defined as the total patient-registry number in each fiscal year, and newly registered patients (newly RPs), defined as the difference between the total patient-registry number in each fiscal year as compared to the previous year. This research helped distinguish sudden TAK outbreaks, involving 590 newly RPs in 2,985 present RPs in just1 year (1984).
Continued epidemiological research has been conducted regarding approximately40 SIDs to clarify the relationship between the annual occurrence of SIDs and annual AP exposure during 1974-2014. This research specifically evaluated vasculitis syndromes such as TAK, Behçet’s disease, Buerger’s disease or thromboangiitis obliterans, granulomatosis with polyangiitis (GPA), and periarteritis nodosa (PAN), as well as connective tissue diseases accompanying autoimmune diseases such as systemic lupus erythematosus (SLE), rheumatoid vasculitis, scleroderma, sarcoidosis, and pemphigus, and a plastic anemia [11]. A 2019 study further evaluated the occurrence of digestive disorders including inflammatory bowel disease, ulcerative colitis (UC), Crohn’s disease (CD), primary biliary cirrhosis (PBC), fulminant hepatitis, and severe acute pancreatitis, as well as interstitial pneumonia, idiopathic thrombocytopenic purpura, amyloidosis and a plastic anemia [12].
We now further report this research with a manuscript concerning muscular intractable diseases such as IDCM, myasthenia gravis, and polymyositis/dermatomyositis (PM/DM) and the disorders TAK, Behçet’s disease, Buerger’s disease, GPA, and PAN, and a plastic anemia, too, which are reported to be associated with these muscular diseases, as cited below. Previous studies have reported on the relation between IDCM and TAK [13], inflammatory dilated cardiomyopathy (DCM) andeosinophilic GPA [14], and autoimmune or autoinflammatory diseases and myocarditis, such as sarcoidosis, Behçet’s disease, eosinophilic GPA, myositis, and SLE [15].
In this research, we examined the relationship between upward peaks of AP released in the Bunkyo-City area of Tokyo, in the whole area of Tokyo Metropolitan, and in Sagamihara City of Kanagawa Prefecture in relation to the increase in the annual number of newly RPs during 1974-2014. The aim of our study was to evaluate the associations between AP exposure peaks and the occurrence of immune-related, principal 40 SIDs including IDCM, MG, PM/DM, vasculitis syndrome, connective tissue diseases, gastroenterological diseases, neuro intractable diseases and so on in Japan. We hypothesized that these immune-related diseases might belong to the class of pollen-induced diseases (PIDs), or “pollen diseases,” because so far accumulated data have suggested that these conditions may be triggered when susceptible patients receive AP exposure that exceed the pollen-responsive threshold of the individuals, and reach a starting line to development of diseases.
Materials and Methods
Since 1974, the Japanese governmental authority (JIDRF) has assigned certificates to SID patients to support their treatment financially following registration in a national database [3]. The homepage of the JIDRF reports the “numbers of recipient certificates issued for specific disease treatment” based on registration beginning in 1974 or 1975, 1983 or 1984, and so on until 2014. The data show the number of presently RPs in the current fiscal year as well as the number of newly RPs relative to the previous fiscal year for all the major SIDs. These increments could be negative if the number of presently RPs in the present fiscal year was smaller than that in the previous fiscal year, which occurs occasionally. Since KD is not included in the list of SIDs, the number of KD patients in every calendar year was downloaded from the homepage of the Department of Public Health at Jichi Medical University [16]. This study was performed in accordance with the ethical principles for medical research outlined in the Declaration of Helsinki 1964 and subsequent revisions (https:// www.wms.net/).
Data on AP release were provided by Dr. Yozo Saito, Dr. Hiroshi Yasueda, and Professor Norio Sahashi. Dr. Saito gathered the AP data from data at the research unit in the Tokyo Medical Dental University Graduate School of Medicine, Bunkyo-City, Tokyo, and Dr. Yasueda surveyed AP data based on the research at the National Hospital Organization Sagamihara National Hospital, Sagamihara, Kanagawa. The AP data in Tokyo Metropolis were collected from 12 sites in Tokyo and were donated by Mr. Hiroshi Kaneko. The AP data were downloaded after administrative information disclosures from the website of the Tokyo Metropolitan Institute of Public Health [17].
In the present study, data of numbers of SID patients in all the Japan were imported into tables in Microsoft Excel. This data was used to create figures of line graphs for each SID. These figures represent annual numbers of presently RPs as well as newly RPs, and the scattered pollen counts in three areas in Japan (the Bunkyo-City area of Tokyo, the whole area of Tokyo Metropolitan, and Sagamihara City in Kanagawa).
A correlation analysis was performed for each SIDs, to evaluate the association between the annual number of newly RPs in each patient-registry year “x” during 1974–2014, and the annual amount of AP levels in Tokyo and Sagamihara, measured in the same year as the patient-registry data. A correlation shift analysis was also performed between the annual number of newly RPs in each patientregistry year “x” between 1975 and 2014 and the annual AP levels in both cities measured “α” years prior to the patient-registry year “x”(“α”=1~20). Correlation coefficients and p values were calculated using the Excel function PEARSON via the method described in the brochure http://imnstir.blogspot.com/2014/04/p.htm. A statistically significant positive correlation was defined as p<0.05. Marginally significant associations that indicated a possible positive tendency (0.05≤p≤0.10) were also reported for reference.
Results
Occurrence of Upward Peaks in the Line Graphs of the Annual Number of Newly RPs for Ten SIDs in Relation to the Annual Levels of AP Scatter
The five line graphs in our figures for muscular SIDs, IDCM (Figure 1), MG (Figure 2), PM/DM (Figure 3), and the disorders TAK, Behçet’s disease, Buerger’s diseas and a plastic anemia (Figure 4), GPA (Figure 5), and PAN (Figure 6) consist of two line graphs visualizing the annual patient-registry data for presently RPs and newly RPs, and three line graphs visualizing the annual amount of AP scatter measured in three geographical areas. Our earlier research report regarding 11 SIDs showed only two line graph indicating change in the numbers of presently RPs and newly RPs for vasculitis syndrome and connective tissue disorders (including TAK, Behçet’s disease, Buerger’s disease, GPA, PAN, SLE, rheumatoid vasculitis, scleroderma, sarcoidosis, pemphigus) and aplastic anemia, occurring between 1974 and 2014 [11].
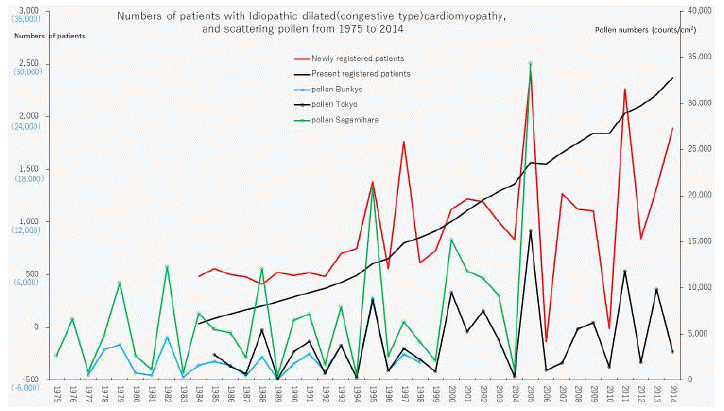
Figure 1: Numbers of patients with idiopathic dilated cardiomyopathy and scattering pollen from 1975 to 2014.
The line graphs for idiopathic dilated cardiomyopathy representing numbers of present registered and newly registered patients in each year, as well as the amount
of pollen scattered in Bunkyo-ku, Metropolitan Tokyo and Sagamihara city during the period from 1975 to 2014.
Numbers of patients are shown on left axes whose scales are black reduced numbers to newly registered patients and (blue actual numbers) to present registered
patients. Pollen numbers are shown on right axes whose scales are counts/cm2.The remainder of the text is the same as in this sentence (Figures 2-6).
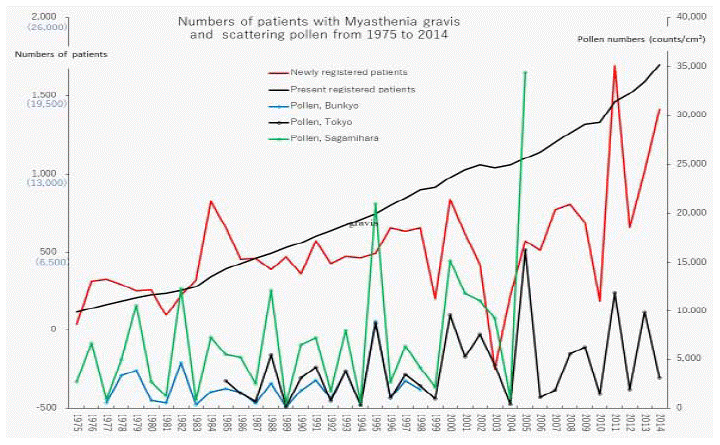
Figure 2: Numbers of patients with myasthenia gravis and scattering pollen from 1975 to 2014.
The line graphs for myasthenia gravis representing numbers of present registered and newly registered patients in each year, as well as the amount of pollen during
the period from 1975 to 2014.
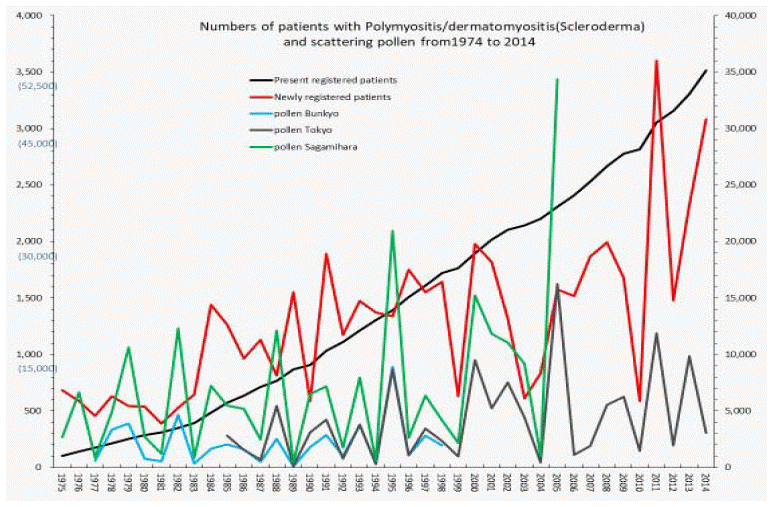
Figure 3: Numbers of patients with polymyositis/dermatomyositis and scattering pollen from 1975 to 2014.
The line graphs for polymyositis/dermatomyositis representing numbers of present registered and newly registered patients in each year, as well as the amount of
pollen during the period from 1974 to 2014.
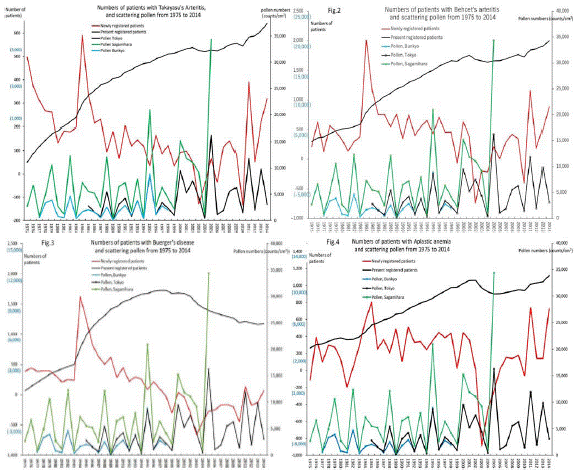
Figure 4: Numbers of patients with Takayasu arteritis,Behçet’s disease Buerger’s diseaseand aplastic anemia, and scattering pollen from 1975 to 2014.
The line graphs for Takayasu arteritis(upper left), Behçet’s disease(upper right), Buerger’s disease(lower left) and aplastic anemia(lower right)representing numbers
of present registered and newly registered patients in each year, as well as the amount of pollen during the period from 1975 to 2014.
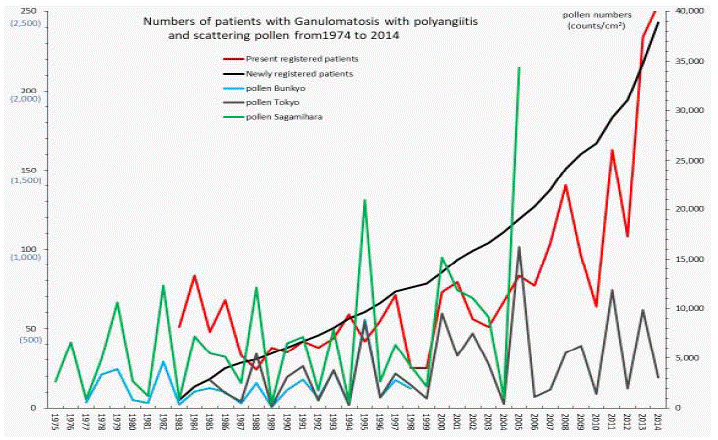
Figure 5: Numbers of patients with granulomatosis with polyangiitis and scattering pollen from 1975 to 2014.
The line graphs for granulomatosis with polyangiitis representing numbers of present registered and newly registered patients in each year, as well as the amount
of pollen during the period from 1975 to 2014.
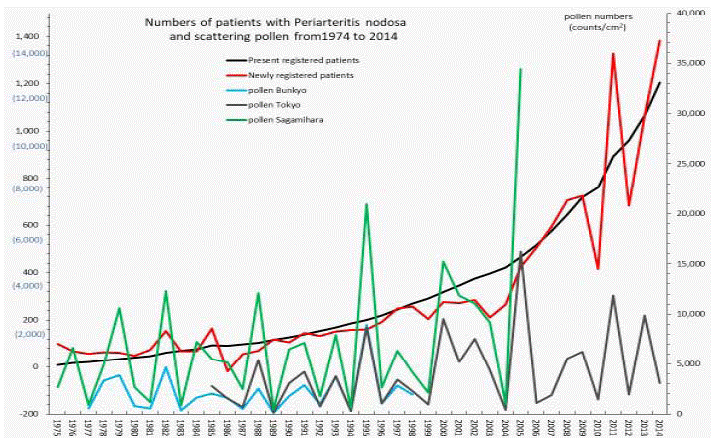
Figure 6: Numbers of patients with periarteritis nodosa and scattering pollen from 1975 to 2014.
The line graphs for periarteritis nodosa representing numbers of present registered and newly registered patients in each year, as well as the amount of pollen
during the period from 1975 to 2014.
IDCM was registered as an SID in Japan starting in 1984, and the annual patient-registry data were then released to the public domain. From 2015-2018, in addition to IDCM, data regarding the numbers of patients with hypertrophic cardiomyopathy and restrictive cardiomyopathy were also newly released to the public domain. From our calculation, the percentages of IDCM, hypertrophic cardiomyopathy, and restrictive cardiomyopathy were 84.7%, 15.1%, and 0.2%, respectively. Between 2015-2018, data regarding patients with PM/DM were also newly released to the public domain, along with an announcement that scleroderma data between 1974 to 2014 had included both scleroderma and PM/DM grouped together because of administrative sorting protocols. Looking at the epidemiological data from 2015 to 2018, separating the number of PM/DM and scleroderma patients, published by the MHLW, the number of PM/ DM and scleroderma patients was almost 50-50 (42.7% and 57.3%, respectively). Therefore, Figure 3 shows the relationship between the total number of patients with PM/DM and scleroderma and the amount of pollen dispersal, and can be interpreted as a graph showing the relationship between the number of PM/DM patients and the amount of pollen dispersal.
Figure 1 shows 11 phasic increments in patient-registry data for newly registered IDCM patients concomitantly with AP scatter increases during1975–2014 (specifically, during 1984-86, 1988, 1990- 91, 1993, 1995, 1997-98, 2000-03, 2005, 2008-09, 2011 and 2013). Simultaneous increases in the occurrence of MG, PM/DM, TAK, Behçet’s disease, Buerger’s disease, a plastic anemia, GPA, and PAN also took place concomitantly, as represented in the line graphs, in relation to upward peaks in annual AP levels in either the above mentioned years or following a 1- or 2-year delay, with the exception of a few peaks that did not follow this pattern (Figure 2,3,4,5 and 6). Lags of 1–2 years (i.e. delayed peaks) were reported for each SID [12]: as observed in the graph for KD in 1987, 1989, 1992, 1994, 1996, 1998, 2006, and 2007 [12].
During the four decades of 1974–2014, we first observed simultaneous peaks of AP scatter and outbreaks of MG, PM/DM, TAK, Behçet’s disease, Buerger’s disease, a plastic anemiaand GPA in 1984-1986 (Figure 2,3,4, and 5). In the case of PAN, earlier concurrent peaks were found in 1982 and 1985 (Figure 6). In Figure 1, we noticed that simultaneous outbreaks of IDCM were quite closely concurrent with seven upwardAPpeaks starting in 1995 and through 2013.As shown in our line graphs (Figure 1-6), the amount of cedar pollen scatter in both Sagamihara City and Bunkyo-City started to increase during 1977-87, showing three distinct peaks (1978-80, 1982, 1984-86). Steady increases in annual numbers of both presently and newly RPs were observed for MG, PM/DM, and PAN (Figure 2,3, and 6), and slow increases and sometimes decreases in those were observed for TAK, Behçet’s disease, Buerger’s disease, and a plastic anemia (Figure 4) starting in 1978-1980 and continuing up to 2014, concurrent with a consecutive series of 13 upward AP peaks in the above mentioned years. For IDCM and GPA, earlier incremental peaks may have been detected around 1980, had the registration begun in 1974. These findings suggest that the occurrence of each SID appeared to start simultaneously and to increase concurrently with pollen scatter in Japan from the latter half of the 1970s until the early 2010s.
Statistical Relationships between the Number of Newly RPs in Each Patient-Registry Year and AP Levels Measured in the Same Year or Prior to the Patient-Registry Year
We examined the statistical correlations between the annual number of newly registered in each patient-registry year “x” (“x”=1975–2014) for six SIDs and the corresponding annual AP levels in Tokyo and Sagamihara, measured in the same year as the patientregistry data “x” as well as measured with a lag of “α” years before the patient-registry year “x” (“α”=1–20). Statistically significant positive correlations were indicated by p values <0.05 (shown in red in the figures), and marginal associations were indicated by p values between 0.05 and 0.100.Reference data of associations with p values slightly greater than p=0.10 are also indicated in (Table 1). Only p values in “α=0-6” are shown which were gotten by this calculation in this (Table 1).
α
IDCM
MG
PM/SM
TAK
GPA
PAN
0
T
5.46519 × 10-⁶
0.030903
0.017807
0.065831
0.04033
S
2.79084 × 10-5
0.207568
0.101602
0.082615
0.001571
1
T
S
0.161155
0.006352
2
T
0.07373
S
0.027477
0.015041
0.009745
3
T
0.102838
0.052622
0.052699
S
0.103648
0.083169
0.001853
4
T
S
0.020711
5
T
S
0.10526
6
T
0.009246
0.003182
0.03275
0.072459
0.009669
S
0.000463
0.000449
0.23617
0.000413
1.65E-05 × 10-5
Table 1: Statistical relationships between the number of newly RPs in each patient-registry year and AP levels measured in the same year or prior to the patient-registry year. A correlation analysis between the annual number of newly registered patients in each patient-registry year “x” (“x”=1975~2014), and the annual amount of airborne pollen levels in Tokyo (T) and Sagamihara (S), measured in the same year as the patient-registry year “x”, and measured “α” years before the patient-registry year “x” (“α”=1~20) , for idiopathic dilated cardiomyopathy, myasthenia gravis, polymyositis/dermatomyositis, Takayasu arteritis,granulomatosis with polyangiitis and periarteritis nodosa. In this Table, the data in the case of “α” =1~20 are shown.
Figure. No
1
2
3
4
4
4
4
5
6
Years of
pollen peaks and
nextyearsIDCM
GM
PM/DM
TAK
Behçet’s disease
Buerger’s disease
aplastic anemia
GPA
PAN
1978-79,1980
*
○
○
○
○
○
○
○
○
1982, 1983
*
○
○
○
○
○
○
○
1984~86,1987
○
○
○
○
○
○
○
○
○
1988,1989
○
○
○
○
○
○
○
○
1990-91, 1992
○
○
○
○
○
○
○
○
○
1993,1994
○
○
○
○
○
1995 1996
○
○
○
○
○
○
○
○
1997-98
○
○
○
○
○
○
○
○
2000~03
○
○
○
○
○
○
○
○
○
2005 2006
○
○
○
○
○
○
○
○
○
2008~09
○
○
○
○
○
○
○
○
○
2011,2012
○
○
○
○
○
○
○
○
2013,2014
○
○
○
○
○
○
○
○
Table 2: Peaks of occurrence of SIDs in Japan between 1974 to 2014. Concurrent occurrence of upward peaks in line graphs for annual numbers of newly registered patients, and for annual levels of airborne pollen scatter, for idiopathic dilated cardiomyopathy, myasthenia gravis, polymyositis/dermatomyositis, Takayasu arteritis,granulomatosis with polyangiitis and periarteritis nodosa.
Our results showed statistically significant correlations between [the number of newly RPs in the patient-registry year “x” (abbreviated as Nos in “x” below in this column)] and the amount of AP exposure measured in Tokyo in the same patient-registry year “x” for IDCM, MG, PM/DM, and PAN. Similarly, significant correlations were shown for IDCM and PAN between Nos in “x” and AP exposure measured in Sagamihara, in the same patient-registry year “x”. It is to be noted that highly graded positive p values were obtained for IDCM because there was the highest level of distinct consistency between the two peaks of the annual number of newly RPs and annual AP levels in both Tokyo and Sagamihara for this outcome (in 1995, 1997, 2000-2003, 2005, 2008-09, 2011 and 2013). Further, regarding IDCM, significant correlations were found between Nos in “x” and the AP exposure measured in Sagamihara, 2 years prior to the patient- registry year “x” reflecting the patient’s peaks in 1997 and 2007. We also found significant correlations for a variety of SIDs between Nos in “x” and AP exposure measured in Tokyo, “α” years prior to the patient-registry year “x” (MG, “α”=6 and 16; PM/DM, “α”=6 and 16; TAK “α”=6 and 16; GPA, “α”=9 and 13; PAN, “α”=6, 9 and 11). Similarly, significant correlations were shown for a variety of SIDs between Nos in “x” and the AP exposure measured in Sagamihara, “α” years prior to the patient-registry year “x” (MG, “α”=6 and 16; PM/DM, “α”=6 and 16; TAK “α”=16; GPA, “α”=2,6,8,9, and 13; PAN, “α”=1, 2, 3, 4, 6, 8, 9, and 11). There were many positive tendencies observed for “α”=0 to 6, 8-9, 11 to 14, 16, and 18, as in part shown in Table 1, which may be part of a pattern of a general correlation between triggering of pollen exposure and occurrence of muscular and vasculitis-related SIDs.
Discussion
Earliest Outbreaks of Multiple SIDs in 1982-86, associated with Increasing AP Release
Airborne pollen levels in Japan began to increase rapidly between the years 1977 and 1987. At this time, the occurrence of KD in Tokyo, Kanagawa, and throughout Japan increased greatly, with triphasic out breaks in the years 1978-80, 1982, and 1984-86 that clearly coincided with triphasic peaks of AP release in Japan. A large number of immune-mediated SIDs, such as IDCM, MG, PM/DM, TAK, Behçet’s disease, Buerger’s disease, and a plastic anemia, showed monophasic outbreaks, whereas GPA and PAN showed biphasic outbreaks associated with increasing AP release in 1982-1986.
The difference in response between KD and the SIDs reported here, including in younger people, maybe understood in the context of the 1.5 million babies born each year at that time. Hence, constitionally allergic infants and/or susceptible to vascular disorders were supposed to be newly diagnosed with KD during the three peaks, within 21.4 months after birth on average [8]. This can potentially be explained by the effects of fast cumulative pollen exposure during rather shorter period different from SIDs’ onset in adults who have been continuing to receive pollen exposure for longer years from birth.
The reasons for the large increase in the release of Japanese cedar pollen, excluding global warming, were explained in our previous paper [6,7,11,12]. Briefly, these reasons include special circumstances such as Japan’s afforestation policy and the repercussions of economic needs following World War II. Other reasons include the increase in abandoned forests due to commercial prioritization.
KD and allergic rhinitis (pollinosis) triggered by pollen exposure were first reported in Japan during the early 1960s, a period ofhigh economic growth and increase in motorization. Cedar pollen release started to increase rapidly, beginning in approximately 1978-79 and continuing until 1988, with the highest peak in 1982 [6, 9,10].
This study confirmed that during the four decades spanning 1974-2014, the upward peak in 1982-1986 was the earliest peak with concurrent outbreaks of a large number of immune-mediated diseases. These include muscle diseases, IDCM, MG, PM/DM, and vasculitis diseases, TAK, Behçet’s disease, Buerger’s disease, GPA, PAN, and a plastic anemia, as reported in this study, as well asautoimmune diseases including connective tissue SIDs and gastroenterological SIDs reported in previous studies [11,12].
Later Outbreaks of Multiple SIDs Associated with Increasing AP Release in 1995, 1997-98, 2000-03, 2005, 2008-09, 2011, and 2013
The simultaneous outbreaks of a large number of immunemediated diseases associated with peaks of AP levels started in 1982- 86 and occurred again in 1996-98 after the largest AP scatter at that time in 1995.This was followed by further outstanding outbreaks of immune-mediated diseases occurring in 2000-03, 2005, 2007-09, 2011, and 2013.Such outbreaks of multiple SIDs associated with increasing AP release are compatible with our observation that the steady increase in annual numbers of both presently and newly RPs for immune-mediated diseases continued up to 2014.
In our study, we hypothesized that a consecutive series of 13 AP peaks in 1978-80, 1982, 1984-86, 1988, 1990-91, 1993, 1995, 1997- 98, 2000-03, 2005(the largest peak so far), 2008-09, 2011, and 2013 produced the cumulative effects of pollen exposure that might trigger immune-mediated diseases, if those cumulative effects overwhelm the immune-reactive threshold (pollen reactivity threshold) of each individual for disease onset.
Statistical Relationships between the Number of Newly RPs in Each Patient-Registry Year and AP Levels Measured in the Same Year as or Prior to the Patient-Registry Year
We examined the relationship between increments in the number of newly RPs with each disease and the amount of AP released in Tokyo and Sagamihara in the same year. Our results showed that the occurrence of IDCM, MG, PM/DM, and PAN was consistently associated with AP release in the same year. In contrast, in our previous cross-correlation(CC) study on the monthly volume of pollen release at Sagamihara in Kanagawa and the change in KD incidence, with a total of 6,000 cases occurring during 144 months between 1991 and 2002, we found a significant positive CC with 9- to 10-month delays following pollen release, a smaller but significant CC with a 3- to 4-month delay, and a further significant positive CC with 21- to 22-month and 33-month delays [8].
The increments in newly RPs with IDCM, GPA, and PAN were also significantly correlated with AP exposure 1-2 years prior to the collection of patient-registry year. Furthermore, associations for AP exposure occurring 6 years prior to the patient-registry year were distinct in the development of MG, PM/DM, TAK, GPA, and PAN. There are many other significant correlations between the numbers of newly RPs with each SID and AP exposure for each (8-16) year prior to the patient-registry year, as described in the Results. It is possible that the accumulation of experiences and memories of pollen exposure in years much earlier than the six years immediately prior to disease onset may effectively promote disease onset. These findings show that the same pollen exposure can lead to vasculitis syndrome in some people, ulcerative colitis in others, and Parkinson’s disease in others. The starting point for disease onset is when pollen exposure in a given year exceeds an individual’s threshold for pollen reactivity. The time from the starting line to the onset of disease is assumed to vary depending on the disease, with some diseases developing immediately in the same year as the starting line, some developing within 1-2 years, and others taking 6 years to develop. Factors that determine the number of years required for the biological process from the starting point to disease onset in an individual may depend on the individual’s genetic predisposition or on disease-specific pathogenic factors. A clinical-epidemiological analysis of these issues is awaited.
Regarding to TAK, between 2002 and 2003, the number of newly RPs began to decrease drastically. (Figure 4) This may be because a considerable number of KD diagnoses had already been made among potentially susceptible individuals between 1978 and 1988; in particular, the pool of female patients at risk of vasculitis may have decreased. Significant statistical results showed that the preliminary TAK group, whose pollen exposure exceeded the threshold for pollen reactivity and who were at the starting line of disease onset, developed Takayasu disease 6 years later. Behçet’s disease, Buerger’s disease, and a plastic anemia also showed a marked decrease in the number of new patients in 2002-2003, as did TAK. Although an association between pathogenesis dynamics of these three diseases and pollen dispersal seems relevant as seen in the graphs and tables (Figure 4) (Table 1), the correlation coefficients over the 40-year period are not significant. For the three vasculitis syndromes, the accumulation of previous pollen exposure exceeded the threshold as a result of the 1982-1984 mass pollen dispersal. Next, in 1984-1985, a remarkable epidemiological fact was exposed: a large number of people in the patient reserve group reacted simultaneously and developed the disease. Therefore, in subsequent years, there was a long period of steady decline with increase in the number of cases compared to the previous year, which lowered the correlation between pollen exposure and the number of patients who developed the disease. A number of affected children with a predisposition to developing Kawasaki disease developed Kawasaki disease after being exposed to large amounts of pollen on three occasions. As a result, it is possible that children with a predisposition to developing vasculitis syndromes may have been prevented from developing other vasculitis syndromes other than Kawasaki disease as they grew into adults. It is noteworthy that the annual dynamics of Takayasu’s disease and Behcet’s disease incidence (Figure 4) are not similar before 1982, but are very similar in the period from 1982 to 2014. Therefore, the exploration of pollen responsiveness and genetic characteristics of patients with these two diseases has the potential to create new academic concepts.
Hypothetical Mechanisms underlying the Development of KD and SIDs Due to Pollen Exposure, as well as the Prevention and Monitoring Processes During the Development of These Diseases
This article presents the first report on the epidemiological correlation between the incidence of muscular SIDs and AP levels. As described in the Introduction, the development of these SIDs is reported to be co-occurring in patients in many papers. Pender et al. published a review that proposed a unifying hypothesis regarding CD8+ T-Cell deficiency, Epstein-Barr virus infection, vitamin D deficiency, and steps to autoimmunity, which described that CD8+ T-cell deficiency is a feature of many chronic autoimmune diseases, including but not limited to multiple sclerosis, rheumatoid arthritis, SLE, systemic sclerosis, DM, PBC, UC, CD, bullous pemphigoid, IDCM, MG, and pernicious anemia [18].
Our study indicated that common substances in pollen cells seemed to be involved in the outbreaks of each SID, accompanied with increased rates of cancer and malignant tumors [19,20] and KD over the course of 40 years in Japan. It is critical to further analyze the relationship between the onset of various SIDs, cancer and malignant tumors, KD, pollinosis, and asthma triggered by AP exposure in a large cohort study.
Clinical observations of symptomatic immune responses and laboratory tests from the earliest stage of disease development or even during the process of disease onset over the course of many years of pollen exposure (starting from childhood, birth, or in utero) are required.
Furthermore, to verify that these SIDs, including autoimmune diseases, are commonly triggered by pollen exposure, we will need to establish routine laboratory tests for useful diagnostic parameters and to develop methods to track disease progression chronologically in the acute phase or early in the disease course and during remission or recurrence. For example, a clinical experimental study on a lymphocyte stimulation test regarding blastoid transformation of lymphocytes sensitized to specific antigenic constituents of pollens, such as Cryj1 and Cryj2, or other substances in SID patients is desirable [21,22]. Alternative methods are required to measure auto antibodies to several putative cross-reactive antigens from pollen in patients with each type of SID.
Currently, it is extremely important to devise experimental systems using animal models with an allergic constitution, so that pollen exposure or pollen immunization in animals can cause DCM, MG, PM/DM, KD, TAK, and other autoimmune diseases such as collagen diseases and inflammatory bowel diseases. The circuit processes by which pollen exposure promotes the development of muscle disease are unknown. We hope therefore that future studies will be conducted using experimental animal models of the four muscle diseases caused by muscle contraction disorders discussed in this paper to verify the experimental fact that pollen exposure promotes the development of muscle diseases.
The immunological signals are transferred as immune-mediated stress information from the mucosal organs via immune pathways to the hypothalamic median eminence rich in histaminergic mast cells, which is the brain center controlling immunological homeostasis.
The problems that remain to be solved are questions regarding the antigen recognition mechanisms involved in triggering the systemic inflammatory conditions induced by pollen exposure in patients with immune-mediated SIDs. Our results suggest that pollen cells, living microparticles with mitochondrial DNA that may affect essential vital activities, lead to reactions in various tissues and cells in the human body.
The following possibilities for disease development based on the seasonal cause of pollen exposure are also considered. When wind-pollinated pollen is dispersed and comes in contact with a host human or animal, the airborne pollutants remain attached to airborne pollen. An important issue is to clarify whether the substances that contact the cellular substances of the immune sensors of human cells and receptors such as toll-like receptors (TLRs) are the protein components of the pollen itself, other macromolecules, low molecular weight components, or airborne substances attached to the pollen.
Signals generated at a new stage of cellular reactivity to the pollen-airborne pollutants complex that exceeds a threshold level can be assumed to be a new stimulus that triggers a further inflammatory response, which is transmitted into the host cells and lands the host at the starting line of the disease pathogenesis mechanism. And we believe that the host will hurry or slow down on the path to the onset of the disease.
During the annual and seasonal periods of heavy pollen dispersal, the intensity of cellular stimulation by these the pollen-airborne pollutants complexes increases, accelerating the rate of disease onset, which may lead to the development of intractable diseases and cancers. The lesson to be learned from epidemiological studies of pollen exposure is that patients undergoing treatment or in remission for incurable diseases or cancer may need to minimize their pollen exposure on a daily basis to prevent recurrence or worsening of symptoms.
Each year, the cellular response to the stimuli generated when the pollen-airborne pollutants complexes come in contact with cell surface materials is inflammatory, but we have hypothesized that there are potentially compensatory biological agents that can also mitigate this inflammation [17].
One such candidate compensatory substance we believe is FTS nonapeptide (serum thymic factor, zinc-free thymulin). It is assumed that upon stimulation of the pollen-airborne pollutants complexes, FTS nonapeptide is presumably excised from some precursor to counter the inflammation involved in pathogenesis.
Regarding IDCM and Myocarditis
Our research had demonstrated that our synthesized FTS nonapeptide has remarkable protective effects in cardiomyopathic hamsters [19,23,24] and in mice with the D-variant of encephalomyocarditis (EMC-D) virus-induced myocarditis and diabetes [25], as well as various other biodefense-related activities in the animals models described below. For example, FTS was first purified from blood as a serum thymic factor by Bach et al. in 1977 [26]. Around 1980, we examined the effect of FTS in a lethal experimental allergic encephalomyelitis model of guinea pigs for a SID (multiple sclerosis) and demonstrated its dramatic preventive effect against lethality [27,28]. Based on these results, we further investigated the diverse immunological and pharmacological activities and biodefense effects of FTS nonapeptides in the above mentioned cardiac models [23,24] and various types of models of organ injury [19,29-42] and aging model [43], and found marked or considerable protective effects in many animal models. We observed that in a pollinosis model, FTS nonapeptide had a strong effect at a dose of 1/1000, similar to that of prednisolone for cedar-pollen-induced late nasal airway responses in actively sensitized guinea pigs [44]. Therefore, we hypothesized that FTS nonapeptide may be a potent intrinsic antagonist that could be used to protect against a wide range of inflammatory and aging processes induced by pollen cell substances, various environmental factors, and extrinsic drugs.
In conclusion, if our findings are confirmed in further prospective studies, patients with SIDs should be recommended to avoid pollen exposure as much as possible for the purpose of preventing the aggravation of symptoms or recurrences, as well as the co-occurrence of other diseases. Pollen-avoidance prophylactic measures include the precaution of wearing safety masks, goggles, and transparent shields during pollen peaks from the early postnatal period, and of installing air cleaners in homes, particularly during seasons with large amounts of pollen release in spring and with small amount of forerunning pollen release of cedar pollen in September to November before next spring. These measures may help avoid or delay the first onset of intractable diseases, including that in young infants who have a potential risk of developing immune-mediated diseases, such as KD and various juvenile SIDs [3].
To prevent the development of cancer and malignant tumors, especially to delay the age of onset in people with cancer families, it is important to reduce cumulative pollen reactivity and extend the time to onset by reducing annual pollen exposure. For this reason, it will be necessary to maintain the above-mentioned lifestyle of wearing masks throughout the year, and to install pollen-cutters.
In addition, we suggest that the application of pollen allergen immunotherapy to reduce pollen reactivity at the level of practical medical care and careful tasting of pollen-containing health foods might be attempted [45].
The aim of our study was to determine the risk of AP exposure and its influence on the occurrence of immune-related diseases such as connective tissue diseases, vasculitis syndrome, and gastroenterological diseases (including inflammatory bowel diseases) in Japan. We hypothesized that these immune-related diseases might also be PIDs or “pollen diseases” because these conditions are assumed to be triggered when susceptible patients are exposed to epigenetic immune-mediated pollen-induced immunological stress induced by pollen cells.
Acknowledgment
Akira Away a deeply appreciates Mr. Koichi Iwata for his elaborate and diligent graphing work and correlation analysis.
Conflicts of Interest
The authors declare no conflict of interest in the preparation of this article.
An Emotion
Hundreds of plant species release their pollen into the air every year during early spring. Why does the risk of new corona infection increase as the pollen count increases? Thinking about the question, there is a prophetic statement in the Bible that mankind became mortal without enjoying eternal life because people who are considered to be our ancestors disobeyed the teachings of God and ate the fruit of the trees. The author thinks of the symbolic analogy, based on the inevitable epidemiological fact that pollen of the same plant seems to be a dominant presence shortening the human life span, which has not been accepted and recognized.
Funding
The authors have never had an opportunity of receiving any research fund in relation to this research.
References
- Akinori Kimura. Molecular genetics and pathogenesis of cardiomyopathy. Journal of Human Genetics. 2016; 61: 41–50. doi:10.1038/jhg.2015.83.
- Yoshinori Seko. From myocardititis to dilated cardiomyopathy -Molecular mechanism of cardiac muscle diorders-Molecular medicine. 1994 edited by Yoshio Yazaki, Yodosha, p108-120.
- http://www.nanbyou.or.jp/entry/ http://www.nanbyou.or.jp/entry/1356
- Hiroyuki Morita. Idiopathic dilated cardiomyopathy. RyoikibetsuShokogun Series No.5 Circulation Syndrome 3rd edition Part 1 Nippon-Rinsho.2019; 125-131.
- Matsuoka S, Tatara K, Nakagawa R, Mori K, Kuroda Y. Tendency toward atopy in Kawasaki disease. Eur. J Pediatr. 1997; 156(1): 30–32.
- Awaya A, Sahashi N. The etiology of Kawasaki disease: does intense release of pollen induce pollinosis in constitutionally allergic adults, while constitutionally allergic infants develop Kawasaki disease?. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie. 2004; 58(2): 136-140. doi:10.1016/J.BIOPHA.2003.08.026.
- Awaya A, Murayama K. Positive correlation between Japanese cedar pollen numbers and the development of Kawasaki Disease. Open Allergy J. 2012; 5: 1-10.
- Awaya A, Nishimura C. A Combination of Cross Correlation and Trend Analyses Reveals that Kawasaki Disease is a Pollen-Induced Delayed-Type Hyper-Sensitivity Disease. International Journal of Environmental Research and Public Health. 2014; 11(3): 2628-2641. doi:10.3390/ijerph110302628.
- AWAYA A. Suppressive influence of seasonal influenza epidemic on Kawasaki disease onset. Nihon Rinsho Men’eki Gakkai kaishi = Japanese journal of clinical immunology. 2016; 39(6): 528-537. doi:10.2177/jsci.39.528.
- Awaya A. Pollen-Induced Diseases No.9; Prevention of Kawasaki Disease by Pollen Avoidance Life: Decrease of Recurrence Cases and Family Cases Are Criteria. YAKUJI NIPPO (Pharmaceutical News). Available online: https:// www.yakuji.co.jp (accessed on 20 September 2017). (In Japanese).
- Akira Awaya. Development of many so-called autoimmune diseases including various vasculitis syndromes may be commonly triggered by pollen exposure. Jacob J Aller Immuno. 2018; 5: 26.
- Awaya A, Kuroiwa Y. A Retrospective study on the relationship between annual airborne pollen levels during four Decades of 1975- 2014 and annual occurrence of ulcerative colitis, Crohn’s disease, primary biliary cirrhosis, fulminant hepatitis, severe acute pancreatitis, interstitial pneumonia, amyloidosis, based on the National Registry Database of Specific Intractable Diseases in Japan. Arch Epidemiol. Public Health. 2019; 1:112
- Goel PK, Moorthy N, Kumar S. The Role of Noninvasive Imaging in Early Diagnosis of Clinically Masked Prepulseless Inflammatory Phase of Takayasu’s Arteritis. Echocardiography. 2012; 29(1): 59-63. doi:10.1111/ j.1540-8175.2011.01581.x.
- Takafumi Nakayama, Shunsuke Murai, Nobuyuki Ohte. Dilated Cardiomyopathy with Eosinophilic Granulomatosis with Polyangiitis in Which Active Myocardial Inflammation Was Only Detected by Endomyocardial Biopsy. Intern Med. 2018; 57(18): 2675-2679. doi: 10.2169/ internalmedicine.0330-17.
- Comarmond C, Cacoub P. Myocarditis in auto-immune or auto-inflammatory diseases. Autoimmunity reviews. 2017; 16(8):811-816. doi:10.1016/j. autrev.2017.05.021.
- https://www.jichi.ac.jp/dph/inprogress/kawasaki/
- http://www.tokyo-eiken.go.jp/
- Michael P Pender CD8+ T-Cell Deficiency, Epstein-Barr Virus Infection, Vitamin D Deficiency, and Steps to Autoimmunity: A Unifying Hypothesis. Autoimmune Dis. 2012; 189096. doi: 10.1155/2012/189096.
- https://www.mdpi.com/1660-4601/17/11/3950/pdf
- https://www.gavinpublishers.com/articles/opinion-article/Archives-of- Environmental-Sciences-and-Environmental-Toxicology/40-specificintractable- diseases-and-24-types-of-cancer-and-malignancies-as-well-askawasaki- disease-may-be-triggered-by-pollen.
- Awaya A, Sugane K, Yamauchi J, Kimura M. Stimulation of lymphocytes of patients administered with a trypsin inhibitor, Trasylol (basic pancreatic trypsin inhibitor pharmaceutical), In vitro with BPTI and other several stimulants. Jpn J Exp Med. 1975; 45(6): 541–549.
- Awaya A, Takeuchi T, Saito T, Yamauchi J, Kimura M. Studies on a patient whose lymphocytes were stimulated In vitro with aprotinin in spite of having no history of therapy with aprotinin preparation (in Japanese). Rinshomeneki. 1979; 10: 1093–1100.
- Kato M, Tsuchiya M, Takeda N, Mochizuki S, Nagano M, Awaya A. The effects of serum thymic factor (FTS) on cardiomyopathic hamster. J Mol Cell Cardiol. 1995; 27: A534 (131).
- Kato M, Tsuchiya M, Takeda N, Mochizuki S, Nagano M, Awaya A. Administration of serum thymic factor (FTS) on cardiomyopathic hamster. J Mol Cell Cardiol. 1996; 28: A66 (260).
- Mizutani M, El-Fotoh M, Awaya A, Matsumoto Y, Onodera T. In vivo administration of serum thymic factor (FTS) prevents EMC-D virus-induced diabetes and myocarditis in BALB/cAJcl Mice. Arch Virol. 1996; 141(1): 73– 83.
- Bach JF, Dardenne M, Pleau JM, Rosa J. Biochemical characterisation of a serum thymic factor. Nature 1977; 266: 55–57.
- Nagai Y, Osanai T, Sakakibara K. Intensive suppression of EAE by serum thymic factor and therapeutic implication for multiple sclerosis. Jpn J Exp Med. 1982; 52: 213–219.
- Akira A. The important role of melanin synthesis and metabolism systems in biodefense mechanisms, and development as foods with function claims of substances having equal bioactivities to a lead compound FTSnonapeptide in drug innovation. Cell 2017. 49: 676–677.
- Kobayashi H, Abe H, Awaya A, Inano H, Shikita M. Serum thymic factor as a radioprotective agent promoting survival after X-irradiation. Experientia. 2005; 46(5): 484-486. doi:10.1007/BF01954239.
- Kobayashi H, Abe H, Ueyama T, Awaya A, Shikita M. Radioprotective effects of serum thymic factor in mice. Radiation research. 1992; 129(3): 351. doi:10.2307/3578036.
- Yamanouchi T, Moromizato H, Kojima S, Shinohara T, Sekino N, Minoda S, et al. Prevention of diabetes by thymic hormone in alloxan-treated rats. European journal of pharmacology. 1994; 257(1-2): 39-46. doi:10.1016/0014- 2999(94)90691-2.
- Tada H, Nakashima A, Awaya A, Fujisaki A, Inoue K, Kawamura K, et al. Effects of thymic hormone on reactive oxygen species-scavengers and renal function in tacrolimus-induced nephrotoxicity. Life sciences. 2002; 70(10): 1213-1223. doi:10.1016/S0024-3205(01)01495-3.
- Kohda Y, Matsunaga Y, Yonogi K, Kawai Y, Awaya A, Gemba M. Protective effect of serum thymic factor, FTS, on cephaloridine-induced nephrotoxicity in rats. Biological & pharmaceutical bulletin. 2005; 28(11): 2087-2091. doi:10.1248/BPB.28.2087.
- Kohda Y, Kawai Y, Iwamoto N, Matsunaga Y, Aiga H, Awaya A, et al. Serum thymic factor, FTS, attenuates cisplatin nephrotoxicity by suppressing cisplatin-induced ERK activation. Biochemical pharmacology. 2005;70(9):1408-1416. doi:10.1016/J.BCP.2005.08.002.
- Inagaki-Ohara K, Kobayashi N, Nishimura H, Sakai T, Matsumoto Y, Hiromatsu K, et al. Effects of a nonapeptide thymic hormone on intestinal intraepithelial lymphocytes in mice following administration of 5-fluorouracil. Cellular immunology. 1996;171(1):30-40. doi:10.1006/CIMM.1996.0169.
- Inagaki-Ohara K, Sakai T, Koya G, Awaya A, Yoshikai Y. A Thymic Hormone Protects Mice from Enteropathy during Acute Graft-versus- Host Disease. Microbiology and Immunology. 1997;41(11):883-889. doi:10.1111/j.1348-0421.1997.tb01945.x.
- Sun X, Yamada H, Yoshihara K, Awaya A, Yoshikai Y. In vivo treatment with a nonapeptide thymic hormone, facteur thymique serique (FTS), ameliorates chronic colitis induced by dextran sulphate sodium in mice. International immunopharmacology. 2007; 7(7): 928-936. doi:10.1016/J. INTIMP.2007.02.014.
- Yara, S.; Kawakami, K.; Awaya, A.; Saito, A. (2001) FTS reduces bleomycininduced cytokine and chemokine production and inhibits pulmonary fibrosis in mice. Clin. Exp. Immunol. 2001; 124: 77– 85.
- Hirai N, Furuyama H, Awaya A, Onuma M. Effect of administration of serum thymic factor (FTS) in calves and rabbits infected with bovine immunodeficiency-like virus. The Journal of veterinary medical science. 1995; 57(2): 307-310. doi:10.1292/JVMS.57.307.
- Onodera T, Yoshihara K, Suzuki T, Tsuda T, Ikeda T, Awaya A, et al. Resistance to Pseudorabies Virus with Enhanced Interferon Production and Natural Killer Cell Activity in Mice Treated with Serum Thymic Factor. Microbiology and Immunology. 1994; 38(1): 47-53. doi:10.1111/j.1348-0421.1994.tb01743.x.
- Onodera T, Taniguchi T, Tsuda T, Yoshihara K, Shimizu S, Sato M, et al. Thymic atrophy in type 2 reovirus infected mice: immunosuppression and effects of thymic hormone. Thymic atrophy caused by reo-2. Thymus. 1991; 18(2): 95-109.
- Miyauchi M, Koshikawa N, Awaya A, Maruyama K. Augmentation with serum thymic factor of suppressive effects on retroviral tumor development in hosts immune to V-onc product. Leukemia. 1997; 11: 213–215.
- Zhao XH, Awaya A, Kobayashi H, Ohnuki T, Tokumitsu Y, Nomura Y. Effects of repeated administrations of facteur thymique sérique (FTS) on biochemical changes related to aging in senescence-accelerated mouse (SAM). Japanese journal of pharmacology. 1990; 53(3): 311-319. doi:10.1254/JJP.53.311.
- Fuchikami J, Chihara M, Takahashi M, Saitou A, Ikezawa K, Fujino A, Awaya A. Effect of serum thymic factor (FTS) on antigen induced early and late phase nasal blockage in a Guinea pig model of allergic rhinitis. J Pharmacol Sci. 2006; 100: 231.
- Awaya A. Medicinal compositions for cancers and intractable diseases. Tokkai 2021-80252 laid open on 2021.(applied on Nov. 19, 2019).
Citation: Awaya A, Kuroiwa Y. Relationship Between Annual Airbornepollen Levels (1974–2014) and the Occurrence of Idiopathic Dilated Cardiomyopathy, Myasthenia Gravis, Polymyositis/Dermatomyositis, and Vasculitis Syndrome Based on the National Registry Database of Specific Intractable Disease in Japan: A Retrospective Study. Austin J Clin Immunol. 2022; 8(1):1048.