
Review Article
Austin J Clin Immunol. 2023; 9(1): 1051.
PD-1/PD-L1 Immunotherapy: Combating Drug Resistance in Breast Cancer
Gisha Rose Antony, Sulfath Thottungal Parambil, Ajeesh Babu Littleflower and Lakshmi Subhadradevi*
Division of Cancer Research, Regional Cancer Centre (Research Centre, University of Kerala), Thiruvananthapuram, India
*Corresponding author: Lakshmi Subhadradevi Additional Professor, Laboratory of Molecular Medicine, Division of Cancer Research, Regional Cancer Centre (Research Centre, University of Kerala), Thiruvananthapuram, Kerala-695011, India
Received: January 12, 2023; Accepted: February 14, 2023; Published: February 21, 2023
Abstract
In recent years, immune-checkpoint blocking therapy targeting the PD-1/PD-L1 axis has advanced tumor immunotherapy to a new level, with positive outcomes in a variety of malignant tumors. Tumors can avoid an antigen-specific T cell immune response by means of PD-1/PD-L1 signaling, which adversely controls T cell-mediated immune response. In clinical practice, it was shown that while some patients did have long-term success with immunotherapy, the majority eventually had resistance to drugs and recurrence. Hence, one of the main challenges that severely restricts the long-lasting benefits and widespread use of PD-1/PD-L1 blocking treatment is both primary and acquired resistance. Therefore, it is high time to understand the mechanisms of resistance for improving anti-PD-1/PD-L1 efficacy. In this review, we describe major signaling pathways that regulate PD-1/PD-L1 axis in cancers as well as the role of PD-1/PD-L1 in breast cancer development and progression. In addition, we further discuss the involvement of PD-1/PD-L1 axis in multi-drug resistance in cancers, which affected breast cancer and other solid tumor response rates and durability to treatment strategies.
Keywords: Breast Cancer; Drug Resistance; Immunotherapy; Immune Checkpoints; PD-1/PD-L1
Introduction
Breast Cancer (BC) is the most common female cancer worldwide and the main cause of death, according to the Global Cancer Statistics 2020, surpassing lung cancer [1]. It is a heterogeneous disease that expresses several key proteins as drug targets [2]. Understanding the heterogeneity of the disease has become essential for treatment planning as there are numerous unique subtypes of BC. Triple-Negative Breast Cancer (TNBC) is defined as BC that does not respond to Her2-targeted therapy or hormone therapy and has the worst prognosis of any subtype due to its aggressive nature [3]. Hence, chemotherapy is the treatment option available for the management of TNBC patients.
The immune system plays an integral role in the onset and spread of cancer, and so novel targeted medicines are available. In the tumor microenvironment, the complex interactions of stromal cells, immune effector cells, tumor cells, and various soluble substances play a crucial role in the development and/ or elimination of the disease. Hence, a significant advancement in the treatment of malignant tumors, including BC, is targeting the immune system, immune checkpoint inhibition in particular [4].
Immune-Checkpoints in Cancer
Immune homeostasis maintenance is essential for the proper functioning and survival of the host. Explicit or uncontrolled immune responses against pathogens and mutated or over expressed self-antigens can lead to autoimmune disorders and inflammatory tissue damage. This is prevented by balancing co-stimulatory and inhibitory signals, collectively known as immune checkpoints, to maintain the breadth and amplitude of the immune response [5]. Figure 1 summarizes these co-stimulatory and inhibitory signals. The immune checkpoint receptors, which include Programmed cell Death receptor-1 (PD-1), T cell Immunoglobulin and ITIM domain (TIGIT), Lymphocyte Activation Gene-3 (LAG3), T cell immunoglobulin-3 (TIM3), Cytotoxic T Lymphocyte-Associated molecule-4 (CTLA-4), and B and T cell Lymphocyte Attenuator (BTLA), have been discovered and researched in relation to cancer in past decades [6]. They go by the name “immune checkpoints,” which refers to the molecules that serve as the gatekeepers of the immune system. Since they are surface molecules, antibodies that block the ligand-receptor interaction can easily reduce their function [7]. Targeting these regulatory mechanisms to increase immune response against tumor cells is a promising strategy of immune checkpoint therapy for cancer [8]. Ipilimumab, a monoclonal antibody against CTLA-4 was the first Immune Checkpoint Inhibitor (ICI) approved by the FDA for the management of advanced melanoma in 2011 [9]. However, only a small fraction of patients benefit from the currently approved ICIs, and resistance following an initial response is a frequent occurrence [10]. However, additional stimulatory and inhibitory pathways have shown promise as immune checkpoint treatment targets, and immunotherapy is now going much farther than this strategy. The most effective immune checkpoint blockade therapy till date is the anti-PD-1/PD-L1 axis, which has been approved to treat a variety of cancer types, including those of the bladder, blood, kidney, skin, liver, and lung [11].

Figure 1: The co-inhibitory/stimulatory signals in the immunologic synapse between antigen presenting cell or tumor cell and T cell in the tumor microenvironment.
The 2018 Nobel Prize in Physiology or Medicine was awarded to the Immune Checkpoint Blockade (ICB) targeting the PD-1/PD-L1 axis, which has been approved for treatment in a variety of solid cancers. Within the tumor microenvironment, the PD-1/PD-L1 pathway regulates the induction and maintenance of immunological tolerance. T cell activation, proliferation, and cytotoxic secretion in cancer are caused by the action of PD-1 and its ligands PD-L1 or PD-L2, which leads to the degeneration of anti-tumor immune responses [12].
Programmed Death Protein-1 (PD-1)
PD-1, also known as CD279, was initially identified in 1992 in interleukin-3 (IL-3)-depleted murine T-cell hybridoma (2B4-11) and murine hematopoietic progenitor (LyD9) cell lines [13]. It is a 55-kDa transmembrane protein with a membrane-permeating domain, an extracellular N-terminal domain (IgV-like), and a cytoplasmic tail with two tyrosine bases situated at the N and C ends, respectively [14]. The amino acid sequence of PD-1 is 15% similar to CD28, 13% similar to the induced T-cell co-stimulator, and 20% similar to CTLA-4 [15]. PD-1 is expressed on the surface of T-cells, B-cells, and NK cells which predominantly controls effector T-cell function in tissues and inhibits their lytic activity in malignancies [16-18]. PD-1 is increased in Dendritic Cells (DCs) by numerous inflammatory stimuli, as it is in T- and B-cells [18]. Although PD-1 is more commonly known as an effectors T cell enhancer in tissues and the tumor microenvironment, it may improve NK cell activity and antibody production by directly and indirectly stimulating PD-1 positive B-cells [19,20]. Due to its potential to be both beneficial and harmful, PD-1 plays two conflicting roles. Owing to its advantageous effects, PD-1 is essential for preserving immunological tolerance and minimizing the regulation of immune responses that are damaging or inefficient. However, PD-1 prevents the proper working of the defense mechanism of the immune system, leading to the growth of malignant cells [21].
Programmed Death Ligand-1 (PD-L1)
PD-L1, a type 1 transmembrane glycoprotein also known as B7-H1 and CD274, is a member of the B7 ligand family. T cells, B cells, macrophages, DC, NK cells, Myeloid-Derived Suppressor Cells (MDSC), and numerous other cell types, including epithelial and endothelial cells, express PD-L1 [22,23]. When tumor cells recognize the PD-1 protein on T cells, PD-L1 protein is up regulated and binds to PD-1 on T cells, causing the T cells to undergo apoptosis and thereby evading the antitumor immunity [24,25]. Therefore, a tumor that over expresses PD-L1 serves to shield itself against CD8+ cytotoxic T cell-mediated cell death. Many studies have reported that the over expression of PD-L1 is associated with an advanced disease state and a poor prognosis in human malignancies such as Non-Small Cell Lung Carcinoma (NSCLC), melanoma, Renal Cell Carcinoma (RCC), gastric, ovarian, and bladder cancer [26-31]. The PD-1/PD-L1 interaction suppresses T-cell activity, decreases cytokine production, causes T-cell lysis, and induces antigen tolerance [32,33]. Indeed, blocking specific immune checkpoints with monoclonal antibodies has been shown to effectively initiate antitumor responses in a variety of solid tumors, including lung, colorectal, ovarian, esophageal, bladder, and BC, in addition to so called "immunogenic" tumor types like melanoma and RCC [34]. Hence, drugs that target PD-1/PD-L1 axis have been developed for immune checkpoint blockade and could trigger responses across various malignancies, providing new hope for the management of cancer. Nevertheless, the clinical response rate to current PD-1/PD-L1 targeted therapies is still low in many patients, and many of them initially respond but eventually develop resistance to these targeted therapies.
PD-1/PD-L1 Pathway in Breast Cancer
By increasing the expression of PD-L1 on the tumor cell surface, the PD-1/PD-L1 inhibitory pathway can be (mis)used to mute the immune system in breast tumors. In our previous study, we have shown that in BC tissues, PD-L1 expression is high more specifically in TNBC subtypes (53.9%) [35]. Increased PD-L1 expression in BC has been associated with increased tumor size, high grade, high proliferation, lymph node status, and HER2-receptor status of the patient [35,36]. According to a recent meta-analysis, BC expressing PD-L1 were more aggressive and had a shorter survival time, whereas TIL expression may be an indicator of a better prognosis [37].
In a study conducted by Schalper et al. (2014) they correlated the PD-L1 mRNA expression with clinicopathological parameters of BC patients. They reported that the PD-L1 mRNA expression on tumor cells is independently associated with increased TIL and better prognosis in BC [38]. Previous study by Mittendorf et al. (2014) also revealed that TNBC has high PD-L1 expression compared to non-TNBC. Furthermore, tissue microarray revealed that breast tumors that were PD-L1 positive had higher TIL in the tumor microenvironment. Their study also revealed that PTEN loss was associated with upregulated PD-L1 expression in BC [39].
PD-1/PD-L1 Inhibitors in Cancer
Since the identification of immune checkpoint proteins, there has been a particular focus on the development of antibodies that inhibit the PD-1/PD-L1 axis. Currently, four mAbs targeting PD-1 and three mAbs targeting PD-L1 have been FDA-approved [40,41] and are undergoing clinical trials for a variety of cancers, which are depicted in Figure 2 and summarized in Table 1, and there are more than fifteen mAbs in clinical development.
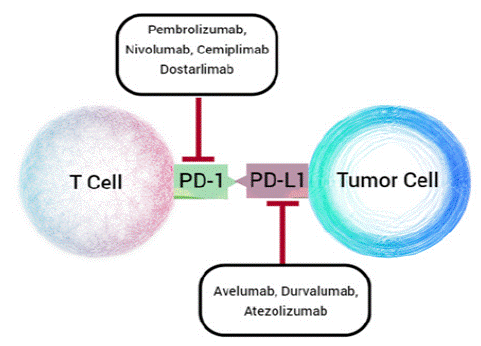
Figure 2: The seven mAbs targeting PD-1 (Pembrolizumab, Nivolumab, Cemiplimab, Dostralimab) and PD-L1 (Avelumab, Durvalumab, Atezolizumab) approved for the treatment of various cancers.
PD-1 Inhibitors
Company
Approval Date
Tumor type
Mechanism
Adverse Effects
References
Pembrolizumab
(MK-3475, Keytruda)Merck Sharp & Dohme Corp.
2014
- Melanoma
- NSCLC
- HNSCC
- MSI-H or dMMR solid tumors
Block the PD-1 receptor pathway by binding to the PD-1 receptor.
- Fatigue
- Musculoskeletal pain
- Decreased appetite
- Pruritus
- Diarrhea
- Nausea
- Rash
- Pyrexia
- Cough
- Dyspnea
- Constipation
- Abdominal pain
- Pneumonia
- Nephritis
- Hepatitis
- Myocarditis
- Colitis
[43-45]
Nivolumab
(OPDIVO, ONO4538, MDX-1106
BMS-936,558)Bristol-Myers Squibb
2014
- Melanoma
- NSCLC
- SCLC
- RCC
- UC
- HNSCC
Selectively block the PD-1 receptor interaction with PD-L1/PD-L2 by binding to PD-1 receptor.
- Increased risk of severe immune mediated inflammation in the lungs, colon, liver and the kidneys.
- Immune-mediated hypothyroidism and hyperthyroidism
- Auto immune diabetes
[46-49]
Cemiplimab
(REGN2810, SAR439684, Libtayo)Sanofi/Regeneron
2018
- CSCC
Binding to the PD-1 receptor and blocks the interaction with PD-L1.
- Immune-mediated pneumonia
- Colitis
- Hepatitis
- Endocrine disorders
- Skin adverse reactions
- Nephritis
- Renal dysfunction
- Fatigue
- Rash
- Diarrhea
[50-52]
Dostarlimab
(Jemperli, TSR-042)GlaxoSmithKline LLC
2021
- MSI-H or dMMRsolid tumors
Binding to the PD-1 receptor and prevents the interaction with PD-L1and PD-L2.
- Fatigue
- Musculoskeletal pain
- Diarrhea
- Nausea
- Rash
- Pyrexia
- Constipation
- hypothyroidism
[41,53-55]
PD-L1 Inhibitors
Company
Approval Date
Tumor type
Mechanism
Adverse Effects
References
Atezolizumab
(MPDL3280A, Tecentriq)Roche Genentech
2016
- NSCLC
- UC
- ES-SCLC
- TNBC
Prevents the interaction of PD-L1 specifically on tumor cells with PD-1 and CD80 receptors and enhances the T-cell mediated antitumor immunity.
- Fatigue/weakness
- Nausea
- Cough
- Dyspnea
- Decreased appetite
- Urinary tract infection
[56-59]
Avelumab
(MSB0010718C, Bavencio)Merck KGaA and Pfizer
2017
- MCC
- RCC
- UC
Inhibits the PD-1/PD-L1 interactions while maintaining the PD-1/PD-L2 pathway.
- Pneumonia
- Hepatitis
- Colitis
- Adrenal insufficiency
- Hyperthyroidism
- Diabetes
- Nephritis
- Fatigue
- Musculoskeletal pain
- Diarrhea
- Nausea
- Infusion-related reactions
- Rash
- Decreased appetite
- Peripheral edema
- Urinary tract infections
[60-60]
Durvalumab
(MEDI4736, Imfinzi)Medimmune/
AstraZenecak2017
- UC
- NSCLC
Block the binding of PD-L1 with PD-1 and CD80 receptors.
- Fatigue
- Musculoskeletal pain
- Constipation
- Decreased appetite
- Nausea
- Peripheral edema
- Urinary tract infections
- Cough
- Dyspnea
- Rash
- Dehydration
[63-65]
NSCLC: Non-Small Cell Lung Cancer; HNSCC: Head & Neck Squamous Cell Carcinoma; SCLC: Small Cell Lung Cancer; RCC: Renal Cell Carcinoma; UC: Urothelial Cancer; CSCC: Cutaneous Squamous Cell Carcinoma; ES-SCLC: Extensive-Stage Small Cell Lung Cancer; MCC: Merkel Cell Carcinoma; MSI-H/dMMR tumors: Microsatellite Instability-High/Mismatch Repair Deficient Tumors.
Table 1: PD-1/PD-L1 inhibitors currently approved by the FDA, along with their indications.
Tumors can avoid anti-PD-1/PD-L1 therapy by creating a hostile Tumor Microenvironment (TME) that reduces the antitumor effectiveness of T cells. Irreversible T cell exhaustion, dysfunction of antigen presentation, inadequate antigen immunogenicity, immunosuppressive TME, and resistance to IFN-γ signaling may all contribute to this [42].
Regulation of PD-1/PD-L1 Pathway in Cancer
Various signaling pathways (Figure 3) are known to be involved in the modulation of the PD-1/PD-L1 axis in cancer and exert a major role in tumorigenesis. Therefore, it is necessary to understand these signaling networks more deeply in order to improve our current knowledge.
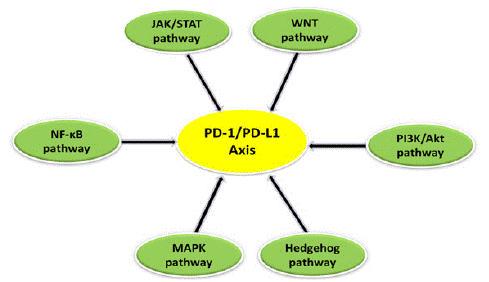
Figure 3: The PD-1/PD-L1 axis is regulated by various signaling pathways such as PI3K/AKT, JAK/STAT, WNT, MAPK, NF-κB and Hedgehog (Hh) pathway.
PI3K/AKT Pathway
PI3K/AKT signaling regulates a number of cellular functions, including apoptosis, proliferation, motility, and metabolism, and also aids in the growth and development of tumors. The lipid phosphatase tumor suppressor PTEN inhibits PI3K activity, and loss of PTEN-mediated PI3K/AKT activation has been shown in a variety of tumor forms [66-68]. According to previous studies, activation of the PI3K/AKT pathway trigger PD-L1 expression in tumor cells by enhancing extrinsic signaling or inhibiting the expression of inhibitory regulators like PTEN. PTEN down regulation may lead to PI3K/AKT activation and enable the up-regulation of PD-L1 [69,70]. Likewise, a study conducted by Zhao et al. (2019) showed that PD-1/PD-L1 blockade could reduce CD8+ T cell apoptosis via the PI3K/AKT/mTOR pathway in Gastrointestinal Stromal Tumors (GIST). They also demonstrated that PI3K, p-PI3K, and p-AKT expression was downregulated in GIST cells following PD-L1 knock down [71]. Furthermore, Wei et al. (2019) showed that colorectal cancer cells with over expression of PD-L1 activate PI3K/AKT in the nucleus [72].
JAK-STAT Pathway
The JAK/STAT pathway is essential for the immune system's coordination and for transferring signals from cell-surface receptors to the nucleus. Many cytokines, growth factors, IFNs, and related molecules use this pathway. Recently, it was shown that the JAK/STAT pathway causes PD-L1 expression in tumors, which may be useful in the treatment of cancer. Zhao et al. (2020) discovered that IFN-γ-mediated PD-L1 over expression in colorectal cancer is regulated by the JAK2/STAT1 signaling pathway and predicts poor patient survival [73]. A study by Ishikawa et al. (2017) showed that JAK/STAT is one of the pathways by which anti-cancer drugs increase the expression of PD-L1 in pancreatic cancer cell lines [74]. Further, it has been shown that silencing and/or inhibiting STAT3 expression reduces PD-L1 expression in NK/T-cell lymphoma (NKTL) [75]. In addition, JAK and STAT3 inhibitors reduced the expression of PD-L1 in breast and lung cancer cells [76-78].
Wnt Pathway
Wnt signaling is a highly conserved signaling system that is essential for regulating organ and embryonic development, as well as the development of cancer. Analysis of gene expression profiles and genome-wide sequencing has shown that deregulated Wnt signaling plays a major role in the proliferation and metastasis of BC [79]. Huang et al. (2021) have shown that the upregulation of PD-L1 in bone marrow-derived fibroblast cells (BMF), stimulated by cancer cells is mediated by the activation of the Wnt/β-catenin signaling pathway, and Wnt/β-catenin signaling inhibitors could inhibit the upregulation of PD-L1 expression in the co-cultured BMFs [80]. Study by Castagnoli et al. (2019) revealed that PD-L1 expression in the stem cell compartment of TNBC is modulated by Wnt signaling [81]. Furthermore, Du et al. (2020) discovered that Wnt ligand-activated EGFR causes the β-catenin/TCF/LEF complex to bind to the CD274 gene promoter region, resulting in PD-L1 expression and immune evasion in glioblastoma [82]. In NSCLC, PD-L1 promotes tumour growth by activating the oncogenic Wnt/ β -catenin pathway and may serve as a potential diagnostic marker [83].
NF-κB Pathway
NF-κB, an important player in immunity and inflammation, is emerging as a positive regulator of PD-L1 expression in many cancers [84]. Notably, previous studies have revealed that NF-κB acts as a key predictor of resistance to immune response in cancer cells in a CRISPR-Cas9-based global screening method [85,86]. A study conducted by Xu et al. (2019) revealed that PD-L1 expression in gastric carcinoma is regulated by NF-κB signaling during Epithelial Mesenchymal Transition (EMT) [87]. Another study has shown that, NF-kB and DNA methylation play a role in controlling PD-L1 expression in NSCLC during the EMT signaling process [88]. Du et al. (2021) discovered a novel mechanism for TGF-β induced PD-L1 transcription and expression regulation via the MRTF-A-NF-κB/p65 axis in NSCLC [89].
MAPK Pathway
The MAPK signaling pathway plays a crucial part in fundamental cellular processes such as cell proliferation, differentiation, development, migration, and apoptosis [90]. Recent studies have focused on the relationship between the PD-1/PD-L1 axis and MAPK pathway in cancer. According to Stutvoet et al. (2019), the MAPK pathway is important in lung adenocarcinoma PD-L1 expression [91]. Combined inhibition of PD-L1 and MAPK signaling may result in long-term responses in advanced melanoma patients [92]. Additionally, Jalali et al. (2019) observed that the anti-PD-L1 antibody reduced the levels of p-P38 and p-ERK in all Hodgkin's Lymphoma (HL) cell lines and that there is an association between the MAPK signaling molecules and PD-L1 antibody in HL cells [93].
Hedgehog (Hh) Pathway
Uncontrolled Hedgehog pathway activation is a powerful oncogenic driving signal that supports a number of hallmarks of cancer, including proliferation, survival, angiogenesis, metastasis, and metabolic reprogramming [94]. A study on pancreatic ductal adenocarcinoma cell lines found that Hh signaling encouraged PD-L1 expression in hypoxic conditions [95]. Furthermore, Chakrabarti et al. (2018) demonstrated that activation of the Hh signaling pathway induces PD-L1 expression and tumour cell proliferation in gastric cancer, rendering the cancer cells immune-resistant. They also suggested that certain patients might benefit from a combination treatment that inhibits Hh signaling and immunological checkpoints [96].
PD-1/PD-L1 Pathway and Multi-Drug Resistance
The main barrier in cancer treatment is intrinsic and acquired multidrug resistance to various chemotherapeutic agents. Drug resistance in cancer is mediated either via transmembrane efflux pumps of ATP-binding cassette family proteins (MDR-1/P-gp) or efflux pump independent factors [35,97,98]. The P-glycoprotein is a 170 kDa protein expressed by the MDR-1 gene, which functions as an efflux pump for many anticancer drugs. Hence, to avoid multi-drug resistance, the over expression of P-gp in cancer cells has been exploited as a therapeutic target [99-101]. However, the regulation of MDR-1 in cancer cells is not well studied. In a study by Liu et al. (2017), a novel connection between PD-L1 and the multidrug resistance protein MDR-1 has been reported. Their study revealed that siRNA-mediated PD-L1 knockdown prevented the over expression of MDR-1/P-gp in the MDA-MB-231 TNBC cell line. Further, they showed that the reverse signal derived from PD-L1 leads to the upregulation of MDR-1 on BC cells through MAPK/ERK and PI3K/AKT pathways [102]. According to a recent study by Lijun et al. (2021), PD-L1 increases cisplatin resistance in gastric cancer cells in the presence of PD-1 through PI3K/AKT-mediated P-gp expression [103]. Targeting the PD-1/PD-L1 axis may increase chemosensitization of aggressive Small Cell Lung Cancer (SCLC), and the poor response to cisplatin treatment may be predicted by cell intrinsic PD-1/PD-L1 signaling [104]. A study conducted by Black et al. (2016) found that breast and prostate cancer cells develop resistance to docetaxel and doxorubicin when incubated with recombinant PD-1. Further, they showed that chemoresistance prompted by the PD-1/PD-L1 axis was suppressed either by inhibition of PD-1/PD-L1 antibody or by PD-L1 gene silencing. Additionally, their study revealed that using an anti-PD-1 antibody to block the PD-1/PD-L1 axis improved treatment with doxorubicin to prevent metastases in a syngeneic mammary orthotopic mouse model of metastatic BC [105]. Therefore, inhibition of the PD-1/PD-L1 axis may also act as a novel strategy to reduce cancer drug resistance and increase chemotherapeutic efficacy in addition to being an efficient immune checkpoint blockade strategy.
PD-L1 as a Biomarker in Cancer
PD-1/PD-L1 targeted immune-checkpoint inhibitors have significantly improved patient outcomes in several types of cancer, despite benefiting only 20-40% of patients [106]. As not all patients may respond favorably to these targeted therapies, the expression of PD-L1 on tumor cells may be considered a predictive biomarker of response to PD-1/PD-L1 checkpoint inhibitors [35,107]. But there isn't a consensus on how to assess PD-L1 expression in terms of choosing the right antibody clone and the cutoff percentage to define PD-L1 negative and positive expression [108]. According to several tests with various cutoffs, patients who have PD-L1 over expression typically respond more forcefully to anti-PD-L1-directed treatment. Patients with PD-L1 over expression melanoma, for instance, have a response rate to anti-PD-1-targeted treatment of 44%–51%, but patients with PD-L1-overexpression in NSCLC have a response rate of 67%–100%. The response rate for PD-L1-negative NSCLC is between 0% and 15%, while it ranges from 6% to 17% for patients with PD-L1-negative melanoma [109]. Patients with a biomarker, for instance, may not benefit from a treatment because the tumor is driven by secondary biologic or molecular alterations. Individuals who test negative for a biomarker may respond to a specific treatment because active biologic pathways can transect, activating a pathway or signal but not activating or over expressing the specific biomarker. PD-L1 has drawbacks, but its dynamic nature demonstrates how the immune system and tumor interact dynamically. As we keep on achieving more knowledge about immunotherapy, we will keep searching for that elusive cure, as we have made incredible progress from the days of conventional chemotherapy for the management of cancer.
Conclusions
BC was traditionally thought to be immunologically "cold," making immunotherapy treatment challenging. However, clinical research and the development of novel medications have demonstrated that immunotherapy treatment has the potential to enhance outcomes for BC patients. The capacity of immune system to restrain itself from attacking healthy cells is a crucial component, which is accomplished via the proteins on immune cells known as "checkpoints," which must be activated (on/off) in order to initiate an immunological response. These checkpoints are occasionally used by BC cells to evade immune system attacks. Drugs that target these checkpoint proteins, help restore the immune response against BC cells. Among which the anti-PD-1/PD-L1 therapy has become a milestone of immunotherapy.
Despite having significant success in the treatment of solid tumors, anti-PD-1/PD-L1 therapy has several drawbacks. The long-lasting sensitivity was only experienced by a limited percentage of participants. Some people who initially responded to the treatment developed acquired resistance in the later stages. Therefore, understanding the causes of anti-PD-1/PD-L1 resistance is essential to developing effective countermeasures. We have compiled the potential role of the PD-1/PD-L1 axis in drug resistance, which affected BC and other solid tumor response rates and durability to treatment strategies in this review article. However, due to the intricacy of antitumor immunity, the known mechanisms are just a small part of the puzzle and differ from person to person, making it difficult to choose patients and develop overcoming tactics.
References
- H Sung, J Ferlay, RL Siegel, M Laversanne, I Soerjomataram, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J Clin. 2021; 71: 209-249.
- K Polyak. Heterogeneity in breast cancer. J Clin Investig. 2011; 121: 3786-3788.
- AA Thike, PY Cheok, AR Jara-Lazaro, B Tan, P Tan, et al. Triple-negative breast cancer: clinicopathological characteristics and relationship with basal-like breast cancer. Mod Pathol. 2010; 23: 123-133.
- AD Hartkopf, FA Taran, M Wallwiener, CB Walter, B Krämer, et al. PD-1 and PD-L1 immune checkpoint blockade to treat breast cancer. Breast Care. 2016; 11: 385-390.
- K Jung, I Choi. Emerging co-signaling networks in T cell immune regulation. Immune Netw. 2013; 13: 184-193.
- JA Marin-Acevedo, B Dholaria, AE Soyano, KL Knutson, S Chumsri, et al. Next generation of immune checkpoint therapy in cancer: new developments and challenges. J Hematol Oncol. 2018; 11: 1-20.
- X He, C Xu. Immune checkpoint signaling and cancer immunotherapy. Cell Res. 2020; 30: 660-669.
- P Sharma, JP Allison. The future of immune checkpoint therapy. Science. 2015; 348: 56-61.
- H Ledford. Melanoma drug wins US approval. Nature. 2011; 471: 561.
- JA Marin-Acevedo, AE Soyano, B Dholaria, KL Knutson, Y Lou. Cancer immunotherapy beyond immune checkpoint inhibitors. J Hematol Oncol. 2018; 11: 1-25.
- A Ribas, JD Wolchok. Cancer immunotherapy using checkpoint blockade. Science. 2018; 359: 1350-1355.
- Y Han, D Liu, L Li. PD-1/PD-L1 pathway: current researches in cancer. Am J Cancer Res. 2020; 10: 727.
- Y Ishida, Y Agata, K Shibahara, T Honjo. Induced expression of PD‐1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. The EMBO J. 1992; 11: 3887-3895.
- BG Neel, H Gu, L Pao. The ‘Shp’ing news: SH2 domain-containing tyrosine phosphatases in cell signaling. Trends Biochem Sci. 2003; 28: 284-293.
- BM Carreno, M Collins. The B7 family of ligands and its receptors: New pathways of costimulation and inhibition of immune responses. Annu Rev Immunol. 2002; 20: 29.
- D Fanoni, S Tavecchio, S Recalcati, Y Balice, L Venegoni, et al. New monoclonal antibodies against B-cell antigens: possible new strategies for diagnosis of primary cutaneous B-cell lymphomas. Immunol Lett. 2011; 134: 157-160.
- M Terme, E Ullrich, L Aymeric, K Meinhardt, M Desbois, et al. IL-18 Induces PD-1–Dependent Immunosuppression in CancerIL-18–Induced Cancer Immunosuppression. Cancer Res. 2011; 71: 5393-5399.
- LM Francisco, VH Salinas, KE Brown, VK Vanguri, GJ Freeman, et al. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J Exp Med. 2009; 206: 3015-3029.
- V Velu, K Titanji, B Zhu, S Husain, A Pladevega, et al. Enhancing SIV-specific immunity in vivo by PD-1 blockade. Nature. 2009; 458: 206-210.
- L Chen, X Han. Anti–PD-1/PD-L1 therapy of human cancer: past, present, and future. J Clin Investig. 2015; 125: 3384-3391.
- A Salmaninejad, V Khoramshahi, A Azani, E Soltaninejad, S Aslani, et al. PD-1 and cancer: molecular mechanisms and polymorphisms. Immunogenetics. 2018; 70: 73-86.
- RM Gibbons Johnson, H Dong. Functional expression of programmed death-ligand 1 (B7-H1) by immune cells and tumor cells. Front Immunol. 2017; 8: 961.
- W Dong, X Wu, S Ma, Y Wang, AP Nalin, et al. The mechanism of anti–pd-l1 antibody efficacy against pd-l1–negative tumors identifies nk cells expressing pd-l1 as a cytolytic effector. Cancer Discov. 2019; 9: 1422-1437.
- SL Topalian, JM Taube, RA Anders, DM Pardoll. Mechanism-driven biomarkers to guide immune checkpoint blockade in cancer therapy. Nat Rev Cancer. 2016; 16: 275-287.
- R Tremblay-LeMay, N Rastgoo, H Chang. Modulating PD-L1 expression in multiple myeloma: an alternative strategy to target the PD-1/PD-L1 pathway. J Hematol Oncol. 2018; 11: 1-16.
- K Azuma, K Ota, A Kawahara, S Hattori, E Iwama, et al. Association of PD-L1 overexpression with activating EGFR mutations in surgically resected nonsmall-cell lung cancer. Ann Oncol. 2014; 25: 1935-1940.
- PA Ott, FS Hodi, C Robert. CTLA-4 and PD-1/PD-L1 blockade: new immunotherapeutic modalities with durable clinical benefit in melanoma patients. Clin Cancer Res. 2013; 19: 5300-5309.
- RH Thompson, H Dong, ED Kwon. Implications of B7-H1 expression in clear cell carcinoma of the kidney for prognostication and therapy. Clin Cancer Res. 2007; 13: 709s-715s.
- L Fashoyin‐Aje, M Donoghue, H Chen, K He, J Veeraraghavan, et al. FDA approval summary: pembrolizumab for recurrent locally advanced or metastatic gastric or gastroesophageal junction adenocarcinoma expressing PD‐L1. The Oncologist. 2019; 24: 103-109.
- C.J. Maine, N.H.A. Aziz, J. Chatterjee, C. Hayford, N. Brewig, L. Whilding, et al. Programmed death ligand-1 over-expression correlates with malignancy and contributes to immune regulation in ovarian cancer. Cancer Immunol Immunother. 2014;63:215-224.
- BA Inman, TJ Sebo, X Frigola, H Dong, EJ Bergstralh, et al. PD‐L1 (B7‐H1) expression by urothelial carcinoma of the bladder and BCG‐induced granulomata: associations with localized stage progression. Cancer. 2007; 109: 1499-1505.
- Y Latchman, CR Wood, T Chernova, D Chaudhary, M Borde, et al. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat Immunol. 2001; 2: 261-268.
- MJ Butte, ME Keir, TB Phamduy, AH Sharpe, GJ Freeman. Programmed death-1 ligand 1 interacts specifically with the B7-1 costimulatory molecule to inhibit T cell responses. Immunity. 2007; 27: 111-122.
- F Schütz, S Stefanovic, L Mayer, A von Au, C Domschke, et al. PD-1/PD-L1 pathway in breast cancer. Oncol Res Treat. 2017; 40: 294-297.
- GR Antony, P Augustine, ST Parambil, AB Littleflower, J Kattoor, et al. Immunohistochemical expression of PD-L1 and MDR1 in breast tumors: association with clinico-pathological parameters and treatment outcome. Clin Exp Med. 2022: 1-11.
- R Sabatier, P Finetti, E Mamessier, J Adelaide, M Chaffanet, HR Ali, et al. Prognostic and predictive value of PDL1 expression in breast cancer. Oncotarget. 2015; 6: 5449-64.
- W Huang, R Ran, B Shao, H Li. Prognostic and clinicopathological value of PD-L1 expression in primary breast cancer: a meta-analysis. Breast Cancer Res Treat. 2019; 178: 17-33.
- KA Schalper, V Velcheti, D Carvajal, H Wimberly, J Brown, et al. In Situ Tumor PD-L1 mRNA Expression Is Associated with Increased TILs and Better Outcome in Breast CarcinomasPD-L1 mRNA Positivity and Outcome in Breast Cancer. Clin Cancer Res. 2014; 20: 2773-2782.
- EA Mittendorf, AV Philips, F Meric-Bernstam, N Qiao, Y Wu, et al. PD-L1 expression in triple-negative breast cancer. Cancer Immunol Res. 2014; 2: 361-370.
- C Bailly, X Thuru, B Quesnel. Combined cytotoxic chemotherapy and immunotherapy of cancer: modern times. NAR cancer. 2020; 2: zcaa002.
- A Cercek, M Lumish, J Sinopoli, J Weiss, J Shia, et al. PD-1 Blockade in Mismatch Repair–Deficient, Locally Advanced Rectal Cancer. N Engl J Med. 2022; 386: 2363-2376.
- Q Lei, D Wang, K Sun, L Wang, Yi Zhang. Resistance mechanisms of anti-PD1/PDL1 therapy in solid tumors. Front Cell Dev Biol. 2020; 8: 672.
- HO Alsaab, S Sau, R Alzhrani, K Tatiparti, K Bhise, et al. PD-1 and PD-L1 checkpoint signaling inhibition for cancer immunotherapy: mechanism, combinations, and clinical outcome. Front Pharmacol. 2017; 8: 561.
- Hughes PE, Caenepell S, Wu LC. Targeted therapy and checkpoint immunotherapy combinations for the treatment of cancer Trends Immunol. 2016; 37: 462.
- J Arranz-Nicolás, M Martin-Salgado, I Adán-Barrientos, R Liébana, M del Carmen Moreno-Ortíz, et al. Diacylglycerol kinase α inhibition cooperates with PD-1-targeted therapies to restore the T cell activation program. Cancer Immunol Immunother. 2021; 70: 3277-3289.
- SL Topalian, M Sznol, DF McDermott, HM Kluger, RD Carvajal, et al. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol. 2014; 32: 1020-30.
- SN Gettinger, FA Shepherd, SJ Antonia, JR Brahmer, LQM Chow, et al. First-line nivolumab (anti-PD-1; BMS-936558, ONO-4538) monotherapy in advanced NSCLC: Safety, efficacy, and correlation of outcomes with PD-L1 status. J Clin Oncol. 2014; 32: 8024-8024.
- S Pasquali, V Chiarion-Sileni, CR Rossi, S Mocellin. Immune checkpoint inhibitors and targeted therapies for metastatic melanoma: a network meta-analysis. Cancer Treat Rev. 2017; 54: 34-42.
- SA Hussain, A Birtle, S Crabb, R Huddart, D Small, et al. From clinical trials to real-life clinical practice: the role of immunotherapy with PD-1/PD-L1 inhibitors in advanced urothelial carcinoma. Eur Urol Oncol. 2018; 1: 486-500.
- D Ogata, T Tsuchida. Systemic immunotherapy for advanced cutaneous squamous cell carcinoma. Curr Treat Options Oncol. 2019; 20: 30.
- A Akinleye, Z Rasool. Immune checkpoint inhibitors of PD-L1 as cancer therapeutics. J Hematol Oncol. 2019; 12: 92.
- A Villani, SS Ocampo-Garza, L Potestio, G Fabbrocini, J Ocampo-Candiani, J Ocampo-Garza, et al. Cemiplimab for the treatment of advanced cutaneous squamous cell carcinoma. Expert Opin Drug Saf. 2022; 21: 21-29.
- PA Shuvo, A Tahsin, MM Rahman, TB Emran. Dostarlimab: The miracle drug for the treatment of colorectal cancer. Ann Med Surg. 2022; 81: 104493.
- H ul Hussain, MH Burney, ST Rehan, MM Hasan. Dostarlimab: A breakthrough in the field of oncology. Ann Med Surg. 2022; 80: 104046.
- A Oaknin, L Gilbert, AV Tinker, J Brown, C Mathews, et al. Safety and antitumor activity of dostarlimab in patients with advanced or recurrent DNA mismatch repair deficient/microsatellite instability-high (dMMR/MSI-H) or proficient/stable (MMRp/MSS) endometrial cancer: interim results from GARNET—a phase I, single-arm study. J Immunother Cancer. 2022; 10: e003777.
- JE Rosenberg, J Hoffman-Censits, T Powles, MS Van Der Heijden, AV Balar, et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet. 2016; 387: 1909-1920.
- RS Herbst, JC Soria, M Kowanetz, GD Fine, O Hamid, MS Gordon, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014; 515: 563-567.
- T Powles, I Durán, MS Van der Heijden, Y Loriot, NJ Vogelzang, et al. Atezolizumab versus chemotherapy in patients with platinum-treated locally advanced or metastatic urothelial carcinoma (IMvigor211): a multicentre, open-label, phase 3 randomised controlled trial. Lancet. 2018; 391: 748-757.
- P Schmid, S Adams, HS Rugo, A Schneeweiss, CH Barrios, et al. Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. N Engl J Med. 2018; 379: 2108-2121.
- CR Heery, G O’Sullivan-Coyne, RA Madan, L Cordes, A Rajan, et al. Avelumab for metastatic or locally advanced previously treated solid tumours (JAVELIN Solid Tumor): a phase 1a, multicohort, dose-escalation trial. Lancet Oncol. 2017; 18: 587-598.
- HL Kaufman, J Russell, O Hamid, S Bhatia, P Terheyden, et al. Avelumab in patients with chemotherapy-refractory metastatic Merkel cell carcinoma: a multicentre, single-group, open-label, phase 2 trial. Lancet Oncol. 2016; 17: 1374-1385.
- MR Patel, J Ellerton, JR Infante, M Agrawal, M Gordon, et al. Avelumab in metastatic urothelial carcinoma after platinum failure (JAVELIN Solid Tumor): pooled results from two expansion cohorts of an open-label, phase 1 trial. Lancet Oncol. 2018; 19: 51-64.
- S Antonia, SB Goldberg, A Balmanoukian, JE Chaft, RE Sanborn, A. Gupta, et al. Safety and antitumour activity of durvalumab plus tremelimumab in non-small cell lung cancer: a multicentre, phase 1b study. Lancet Oncol. 2016; 17: 299-308.
- C Massard, MS Gordon, S Sharma, S Rafii, ZA Wainberg, J Luke, et al. Safety and efficacy of durvalumab (MEDI4736), an anti–programmed cell death ligand-1 immune checkpoint inhibitor, in patients with advanced urothelial bladder cancer. J Clin Oncol. 2016; 34: 3119.
- SS Chang. Re: Safety and efficacy of durvalumab (MEDI4736), an anti-programmed cell death ligand-1 immune checkpoint inhibitor, in patients with advanced urothelial bladder cancer. J Urol. 2018; 199: 341-342.
- J Bai, Z Gao, X Li, L Dong, W Han, J Nie. Regulation of PD-1/PD-L1 pathway and resistance to PD-1/PD-L1 blockade. Oncotarget. 2017; 8: 110693-110707.
- RJ Kim, E Bae, YK Hong, JY Hong, NK Kim, et al. PTEN loss-mediated Akt activation increases the properties of cancer stem-like cell populations in prostate cancer. Oncology. 2014; 87: 270-279.
- A Carnero, JM Paramio. The PTEN/PI3K/AKT pathway in vivo, cancer mouse models. Front Oncol. 2014; 4: 252.
- J Chen, C Jiang, L Jin, X Zhang. Regulation of PD-L1: a novel role of pro-survival signalling in cancer. Ann Oncol. 2016; 27: 409-416.
- ZY Hu, WY Huang, L Zhang, B Huang, SC Chen, et al. Expression of AKT and p-AKT protein in lung adenocarcinoma and its correlation with PD-L1 protein and prognosis. Ann Transl Med. 2020; 8: 1172.
- R Zhao, Y. Song, Y. Wang, Y. Huang, Z. Li, Y. Cui, et al. PD‐1/PD‐ L1 blockade rescue exhausted CD8+ T cells in gastrointestinal stromal tumours via the PI3K/Akt/mTOR signalling pathway. Cell Prolif. 2019;52:e12571.
- F Wei, T Zhang, SC Deng, JC Wei, P Yang, et al. PD-L1 promotes colorectal cancer stem cell expansion by activating HMGA1-dependent signaling pathways. Cancer Lett. 2019; 450: 1-13.
- T Zhao, Y Li, J Zhang, B Zhang. PD‑L1 expression increased by IFN‑γ via JAK2‑STAT1 signaling and predicts a poor survival in colorectal cancer. Oncol Lett. 2020; 20: 1127-1134.
- T Ishikawa, T Okayama, K Oka, K Mizushima, T Yasuda, et al. The JAK/STAT pathway is involved in the upregulation of PD-L1 expression in pancreatic cancer cell lines. Oncol Rep. 2017; 37: 1545-1554.
- TL Song, ML Nairismägi, Y Laurensia, JQ Lim, J Tan, et al. Oncogenic activation of the STAT3 pathway drives PD-L1 expression in natural killer/T-cell lymphoma. Blood. 2018; 132: 1146-1158.
- M Chen, B Pockaj, M Andreozzi, MT Barrett, S Krishna, et al. JAK2 and PD-L1 amplification enhance the dynamic expression of PD-L1 in triple-negative breast cancer. Clin Breast Cancer. 2018; 18: e1205-e1215.
- M Shen, Z Xu, W Xu, K Jiang, F Zhang, et al. Inhibition of ATM reverses EMT and decreases metastatic potential of cisplatin-resistant lung cancer cells through JAK/STAT3/PD-L1 pathway. J Exp Clin Cancer Res. 2019; 38: 149.
- S Ikeda, T Okamoto, S Okano, Y Umemoto, T Tagawa, et al. PD-L1 is upregulated by simultaneous amplification of the PD-L1 and JAK2 genes in non–small cell lung cancer. J Thorac Oncol. 2016; 11: 62-71.
- X Xu, M Zhang, F Xu, S Jiang. Wnt signaling in breast cancer: biological mechanisms, challenges and opportunities. Mol Cancer. 2020; 19: 165.
- T Huang, F Li, X Cheng, J Wang, W Zhang, B Zhang, et al. Wnt inhibition sensitizes PD-L1 blockade therapy by overcoming bone marrow-derived myofibroblasts-mediated immune resistance in tumors. Front Immunol. 2021; 12: 619209.
- L Castagnoli, V Cancila, SL Cordoba-Romero, S Faraci, G Talarico, et al. WNT signaling modulates PD-L1 expression in the stem cell compartment of triple-negative breast cancer. Oncogene. 2019; 38: 4047-4060.
- L Du, JH Lee, H Jiang, C Wang, S Wang, et al. β-Catenin induces transcriptional expression of PD-L1 to promote glioblastoma immune evasion. J Exp Med. 2020; 217: e20191115.
- Y Ma, R Marinkova, M Nenkov, L Jin, O Huber, et al. Tumor-Intrinsic PD-L1 Exerts an Oncogenic Function through the Activation of the Wnt/β-Catenin Pathway in Human Non-Small Cell Lung Cancer. Int J Mol Sci. 2022; 23: 11031.
- F Antonangeli, A Natalini, MC Garassino, A Sica, A Santoni, et al. Regulation of PD-L1 expression by NF-κB in cancer. Front Immunol. 2020; 11: 584626.
- RT Manguso, HW Pope, MD Zimmer, FD Brown, KB Yates, et al. In vivo CRISPR screening identifies Ptpn2 as a cancer immunotherapy target. Nature. 2017; 547: 413-418.
- D Pan, A Kobayashi, P Jiang, L Ferrari de Andrade, RE Tay, et al. A major chromatin regulator determines resistance of tumor cells to T cell–mediated killing. Science. 2018; 359: 770-775.
- D Xu, J Li, RY Li, T Lan, C Xiao, et al. PD-L1 expression is regulated by NF-κB during EMT signaling in gastric carcinoma. Onco Targets Ther. 2019; 12: 10099.
- A Asgarova, K Asgarov, Y Godet, P Peixoto, A Nadaradjane, et al. PD-L1 expression is regulated by both DNA methylation and NF-kB during EMT signaling in non-small cell lung carcinoma. Oncoimmunology. 2018; 7: e1423170.
- F Du, X Qi, A Zhang, F Sui, X Wang, et al. MRTF-A-NF-κB/p65 axis-mediated PDL1 transcription and expression contributes to immune evasion of non-small-cell lung cancer via TGF-β. Exp Mol Med. 2021; 53: 1366-1378.
- AS Dhillon, S Hagan, O Rath, W Kolch. MAP kinase signalling pathways in cancer. Oncogene. 2007; 26: 3279-3290.
- TS Stutvoet, A Kol, EG de Vries, M de Bruyn, RS Fehrmann, et al. MAPK pathway activity plays a key role in PD‐L1 expression of lung adenocarcinoma cells. J Pathol. 2019; 249: 52-64.
- A Ribas, A Algazi, PA Ascierto, MO Butler, S Chandra, et al. PD-L1 blockade in combination with inhibition of MAPK oncogenic signaling in patients with advanced melanoma. Nat Commun. 2020; 11: 1-10.
- S Jalali, T Price-Troska, C Bothun, J Villasboas, HJ Kim, et al. Reverse signaling via PD-L1 supports malignant cell growth and survival in classical Hodgkin lymphoma. Blood Cancer J. 2019; 9: 1-9.
- S. Grund-Gröschke, G. Stockmaier, F.J.C.C. Aberger and Signaling. Hedgehog/GLI signaling in tumor immunity-new therapeutic opportunities and clinical implications. Cell Commun Signal. 2019;17:1-9.
- H Onishi, A Fujimura, Y Oyama, A Yamasaki, A Imaizumi, et al. Hedgehog signaling regulates PDL-1 expression in cancer cells to induce anti-tumor activity by activated lymphocytes. Cell Immunol. 2016; 310: 199-204.
- J Chakrabarti, L Holokai, L Syu, NG Steele, J Chang, et al. Hedgehog signaling induces PD-L1 expression and tumor cell proliferation in gastric cancer. Oncotarget. 2018; 9: 37439.
- Z Zhong, DM Virshup. Wnt signaling and drug resistance in cancer. Mol Pharmacol. 2020; 97: 72-89.
- Q Cui, JQ Wang, YG Assaraf, L Ren, P Gupta, et al. Modulating ROS to overcome multidrug resistance in cancer. Drug Resist Updat. 2018; 41: 1-25.
- J Chen, L Lu, H Wang, L Dai, P Zhang. PKD2 mediates multi-drug resistance in breast cancer cells through modulation of P-glycoprotein expression. Cancer Lett. 2011; 300: 48-56.
- JA Endicott, V Ling. The biochemistry of P-glycoprotein-mediated multidrug resistance. Annu Rev Biochem. 1989; 58: 137-171.
- MM Gottesman, T Fojo, SE Bates. Multidrug resistance in cancer: role of ATP–dependent transporters. Nat Rev Cancer. 2002; 2: 48-58.
- S Liu, S Chen, W Yuan, H Wang, K Chen, et al. PD-1/PD-L1 interaction up-regulates MDR1/P-gp expression in breast cancer cells via PI3K/AKT and MAPK/ERK pathways. Oncotarget. 2017; 8: 99901.
- L Wu, S Cai, Y Deng, Z Zhang, X Zhou, et al. PD-1/PD-L1 enhanced cisplatin resistance in gastric cancer through PI3K/AKT mediated P-gp expression. Int Immunopharmacol. 2021; 94: 107443.
- F Yan, J Pang, Y Peng, JR Molina, P Yang, et al. Elevated cellular PD1/PD-L1 expression confers acquired resistance to cisplatin in small cell lung cancer cells. PloS one. 2016; 11: e0162925.
- M Black, IB Barsoum, P Truesdell, T Cotechini, SK Macdonald-Goodfellow, et al. Activation of the PD-1/PD-L1 immune checkpoint confers tumor cell chemoresistance associated with increased metastasis. Oncotarget. 2016; 7: 10557-10567.
- AA Davis, VG Patel. The role of PD-L1 expression as a predictive biomarker: an analysis of all US Food and Drug Administration (FDA) approvals of immune checkpoint inhibitors. J Immunother Cancer. 2019; 7: 1-8.
- H Li, PA van der Merwe, S Sivakumar. Biomarkers of response to PD-1 pathway blockade. Br J Cancer. 2022; 126: 1663-1675.
- S Gandini, D Massi, M Mandalà. PD-L1 expression in cancer patients receiving anti PD-1/PD-L1 antibodies: A systematic review and meta-analysis. Crit Rev Oncol Hematol. 2016; 100: 88-98.
- SP Patel, R Kurzrock. PD-L1 Expression as a Predictive Biomarker in Cancer ImmunotherapyPD-L1 IHC as a Predictive Biomarker in Cancer Immunotherapy. Mol Cancer Ther. 2015; 14: 847-856.