
Short Commentary
Austin J Clin Immunol. 2023; 9(1): 1053.
High Frequency of Monoclonal B-cell Lymphocytosis with a High Prevalence of Biclonal Cases in the Colombian Population
Celis MA1,2, Navarro Y3, Serrano N4, Calderón Dayro3, Martínez D1 and Nieto WG3*
1University of Santander, Faculty of Medical and Health Sciences, Masira Research Institute, Bucaramanga, Colombia
2Universidad del Valle, Faculty of Health, Ph.D. in Biomedical Sciences, Cali, Colombia
3International Hospital of Colombia, Department of Pathology, FCV Translational Biomedical Research Group, Floridablanca, Colombia
4International Hospital of Colombia, Research Department, FCV Translational Biomedical Research Group, Floridablanca, Colombia
*Corresponding author: Nieto WGDepartment of Pathology, International Hospital of Colombia, 681011, Piedecuesta, Colombia, Colombia
Received: February 16, 2023; Accepted: March 23, 2023; Published: March 30, 2023
Abstract
The World Health Organization (WHO) defines monoclonal B-Cell Lymphocytosis (MBL) as the presence in Peripheral Blood (PB) of monoclonal populations of B-Lymphocytes (BL) of up to 5x109/L, with the phenotype of Chronic Lymphocytic Leukemia (CLL) typical, CLL atypical, or non-CLL, in the absence of B symptoms or clinical manifestations related to chronic B-Cell Lymphoproliferative Disorders (B-CLPD). It is classified as Low Count (LC) (<500 clonal BL/μL) and High Count (HC) (>500 clonal BL/μL) [1]. It has been described in up to 12% of the healthy adult population [1], a proportion that increases in relatives of patients with CLL/Small Lymphocytic Lymphoma (SLL) to around 15% [2]. It is usually a more frequent entity (28.5%) in people with Hepatitis C virus (HCV) [3] infection, and it tends to persist for a long time (90% of cases) with a progression rate of the MBL-HC to clinical manifest B-CLPD between 1-4% per year [1,2]. Currently, it continues to be an entity under study since its possible evolution to CLL or another B-CLPD is clearly unknown [4].
The few studies carried out in Latin America have described a heterogeneous frequency of presentation of MBL. A study carried out in Mexico in the general population, studying the rearrangements of immunoglobulin heavy chains (IgH) identified a prevalence of 9.4% [5], and another study in Brazil identified 10.5% in a population of Japanese descent using the Cytometry technique. of multiparametric flow [6] and in Colombia, the only study carried out to date, identified 2% of MBL in relatives of patients with CLL [7].
The present study determines the frequency of MBL in the healthy adult population (>40 years) of northeastern Colombia using high-sensitivity multiparametric flow cytometry using the screening for CLPD defined by Euro Flow [8] using the protocol described in Salamanca [9]. Subsequently, the clonal population is characterized according to immunophenotype and/or cytogenetics, possible infectious agents related to the presence of MBL are identified; and it is verified if within two years after the identification of clone B, changes are detected in them.
Of 200 individuals analyzed, 85 were men (42.5%) and 115 women (57.5%) with a mean age of 53 years (40-84 years); in which a frequency of MBL of 10% (20/200) was identified, all MBL-LC, which is like that described in other studies from Western countries [3,5,6,10]. Participants with clonal populations range in average age from 61 years (40-81 years), being more frequent in men (60%), without showing significant differences as evidenced by other authors [5,10].
90% of the MBL identified are CLL type (18/20) and it is striking that 50% of the cases correspond to biclonal cases (10/20), a much higher percentage than that identified to date [11,12], which suggests that we are facing oligoclonal expansions of BL, possibly produced in response to past antigenic stimuli and a reflection of the individual's immunosenescence. Although the dynamic difference between biclonality and monoclonality of MBL is largely unknown, it has been shown in 7-year follow-ups that in most cases with more than one clonal population, this can vary, reflecting competition and natural selection among coexisting clones, which has been further supported by changes in the VDJ sequences of the expanded BL of most of these cases, together with the progressively decreasing rate of oligoclonality from MBL-LC to MBL-HC and CLL [13].
The number of MBL/μL on average was 5.8 with a median of 0.41 (0.14-50.4/μL). Statistically significant differences (p=0.0014) are observed between the frequency of MBL and the age of the participants, evidencing a gradual increase with increasing age, which has already been reported by other researchers [2,6]; It is important to highlight that, unlike other studies, the size of clone B decreases with age (Figure 1), contrasting with what has been reported so far [6,10]. When comparing the participants with and without MBL, significant differences were observed in the absolute count of leukocytes/μL (p=0.047), evidencing a decrease in the absolute number of leukocytes ( ̴6504/μL) in individuals with MBL populations, a phenomenon not previously described [6,14], which could suggest a more pronounced level of aging that could be related to the increase in infectious complications [14]. When evaluating the absolute number of the different leukocyte subpopulations (granulocytes, CD4/CD8 TL, BL, NK cells, lymphoplasmocytes, plasmacytoid and monocytoid dendritic cells) and comparing them between adults with MBL and without MBL, only statistically significant differences were observed with lymphoplasmocytes (p=0.024), these being of greater numerical size (10/μL) in the MBL population. Regarding these results, other authors report increases in TL and NK cell populations in patients with MBL [6], which we did not show. Despite this, the slight but significant increase in the absolute number of lymphoplasmacytes in PB for the MBL population could support the hypothesis that MBL-LC is somehow also related to immune cell dysregulation, aging, chronic antigenic stimulation [15] and senescence that helps suppress the formation of cancer cells.
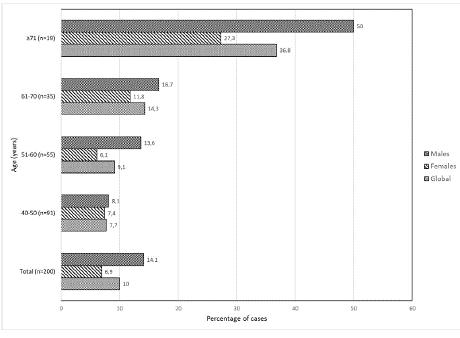
Figure 1: Frequency of MBL related to gender and age.
NS: Not Statistically Significant.
*Statistically significant difference (P<0.05)
All the individuals with MBL populations present IgG antibodies against Herpes Simplex Virus 1 (HSV-1) and Varicella Zoster (VZV), however, no significant differences were found with these and with the other viruses studied. Due to the low percentage of seronegative individuals for HSV-1 and VZV, it is recommended to carry out new studies with a larger number of participants to rule out a possible association. Other works have found a relationship between MBL and HCV, since this, in addition to being a lymphotropic virus, is believed to play an important role in the pathogenesis of some B-CLPD [3]. The fact that these differences were not observed significantly in the present study can surely be attributed to the fact that, in the total population, only 0.7% had antibodies against HCV.
As a curious fact, we found significant differences (p=0.01) between the presence of MBL populations and alcohol consumption, identifying a higher frequency of consumption in the population without MBL (p=0.01), but none of the participants stated that they were in a degree of alcoholism. No study so far has reported significant differences for this variable [2,3,6]. Strangely and coincidentally, studies carried out in people with clinically manifest hematological malignancies have found that alcohol consumption is associated with a reduced risk of these, mainly in Non-Hodgkin Lymphomas (NHL), the mechanisms that explain the decrease remain uncertain. largely unknown, but chronic low-dose ethanol exposure has been shown to inhibit mTOR signaling in lymphocytes with significant repression of cap-dependent translation, thereby reducing the tumorigenicity of NHL [16]. A similar phenomenon could explain the lower frequency of MBL in individuals with frequent alcohol consumption.
Among other associations identified when comparing the participants with and without MBL populations, we found significant differences between the retired population or those who perform work that does not require hours with those with a labor contract (p=0.01), as expected, since the MBL occurs more frequently in older people. Exposure to strange agents such as radiation, dust, and/or chemical substances has been less frequent in the population with MBL (p=0.022), so it would be ruled out that these variables affect the presence of MBL in these subjects.
The results were obtained by dichotomizing the responses to the lifestyle questionnaire and the serology results, with their subsequent pairing using gender, age, and absolute lymphocyte count, using conditional logistic regression to establish the association between the presence of MBL and the dichotomized question, it is found that low consumption of sausages (p=0.010), cheeses and dairy products (p=0.038) are associated with a higher frequency of MBL (Table 1), which had not been previously described for this condition is not usually related to other B-CLPD either [17].
Evaluateditem
MBL populationn(%)
Non-MBL populationn(%)
Total
n(%)OR (95% CI, P-value)
Consumption of sweets
At least one day of the week
3 (6.8)
41 (93.2)
44 (100)
Reference
no day a week
6 (13.6)
38 (86.4)
44 (100)
2.00 (0.50-8.00, p=0.327)
Total
9 (10.2)
79 (89.8)
88
Sausageconsumption
no day of the week
15 (16.7)
75 (83.3)
90 (100)
Reference
At least one day a week
3 (3.3)
87 (96.7)
90 (100)
0.14 (0.03-0.63, p=0.010)
Total
18 (10.0)
162 (90.0)
180
Consumption of olive oil and nuts
One or more days a week
7 (9.1)
70 (90.9)
77 (100)
Reference
None
11 (14.3)
66 (85.7)
77 (100)
1.57 (0.61-4.05, p=0.350)
Total
18 (11.7)
136 (88.3)
154
Consumption of freshfruit
Three or more days a week
4 (6.6)
57 (93.4)
61 (100)
Reference
less than 2 days a week
4 (6.6)
57 (93.4)
61 (100)
1.00 (0.25-4.00, p=1.000)
Total
8 (6.6)
114 (93.4)
122
Frequentdrug use
No
7 (7.4)
88 (92.6)
95 (100)
Reference
Yes
11 (11.6)
84 (88.4)
95 (100)
1.67 (0.61-4.59, p=0.323)
Total
18 (9.5)
172 (90.5)
190
Fishconsumption
At least one day of the week
6 (9.2)
59 (90.8)
65 (100)
Reference
no day of the week
4 (6.2)
61 (93.8)
65 (100)
0.67 (0.19-2.36, p=0.530)
Total
10 (7.7)
120 (92.3)
130
Meatconsumption
Two or fewer days a week
9 (12.3)
64 (87.7)
73 (100)
Reference
Three or more days a week
8 (11.0)
65 (89.0)
73 (100)
0.87 (0.32-2.41, p=0.796)
Total
17 (11.6)
129 (88.4)
146
Consumption of eggs and poultry
Three or more days a week
2 (5.6)
34 (94.4)
36 (100)
Reference
Two or fewer days a week
6 (16.7)
30 (83.3)
36 (100)
3.00 (0.61-14.86, p=0.178)
Total
8 (11.1)
64 (88.9)
72
Cheese and dairyconsumption
Three or more days a week
2 (3.0)
65 (97.0)
67 (100)
Reference
Two or fewer days a week
10 (14.9)
57 (85.1)
67 (100)
5.00 (1.10-22.82, p=0.038)
Total
12 (9.0)
122 (91.0)
134
Vegetable and vegetable consumption
Three or more days a week
4 (9.3)
39 (90.7)
43 (100)
Reference
Two or fewer days a week
3 (7.0)
40 (93.0)
43 (100)
0.67 (0.11-3.99, p=0.657)
Total
7 (8.1)
79 (91.9)
86
Consumption of legumes
Three or more days a week
11 (11.3)
86 (88.7)
97 (100)
Reference
Two or fewer days a week
9 (9.3)
88 (90.7)
97 (100)
0.82 (0.34-1.97, p=0.655)
Total
20 (10.3)
174 (89.7)
194
Consumption of bread, cereals, and tubers
Three or more days a week
5 (6.6)
71 (93.4)
76 (100)
Reference
Two or fewer days a week
9 (11.8)
67 (88.2)
76 (100)
1.80 (0.60-5.37, p=0.292)
Total
14 (9.2)
138 (90.8)
152
A statistically significant difference (P<0.05), (Supplementary Methods). n: number of individuals, the number of individuals varies as a product of the pairing made (%): percentage of individuals; OR: Odds Ratio; CI: Confidence Interval.
Table 1: Association between nutritional factors in subjects with and without MBL matched for age, gender, and lymphocyte count.
On the other hand, FISH studies performed on separate cells do not yield conclusive results. In a participant with a non-CLL immunophenotype, IGH rearrangement evaluation was performed, finding fusions in more than 20% of the hybridized cells; however, the number of cells obtained for this case is less than necessary to be conclusive. In another individual, with CLL-immunophenotype, MLL rearrangements are observed in 24.6% of the cells analyzed. These cytogenetic alterations for rearrangements in MLL and IGH suggest damage that very possibly does not affect genes of greater relevance to trigger uncontrolled clonal proliferation. [10]
After two years of monitoring individuals with MBL populations, we found that in all of them, the clonal population of BL persists and its numerical size increases in 43% of cases; however, none evolve to MBL-HC or another B-CLPD. No significant differences are observed between the absolute number of clonal BL/μL at the start and after follow-up (p=0.790) (Figure 2), which leads to the conclusion that, similar to that observed in other investigations on MBL-LC, probably in these individuals, the presence of clones is not a preleukemic condition and could only be a sign of restrictions in the immunological repertoire that occur with age, immunosenescence and/or constant antigenic stimuli that justify their generation [18]. However, in future follow-ups of individuals with MBL, genetic instability assessments should be considered, which allow determining the presence of new cytogenetic abnormalities, which is considered a prognostic factor for clonal progression [4].
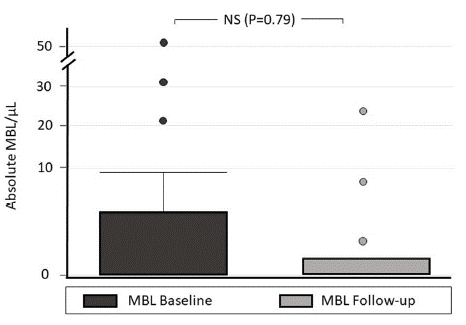
Figure 2: Initial absolute count of MBL cells and after two years of follow-up.
NS: Not Statistically Significant (P> 0.05).
Funding
This project was funded by the Colombian Ministry of Science, Technology, and Innovation. The University of Santander funded the publication fees of this article.
References
- Swerdlow SH, Campo E, Pileri SA, Harris NL, Stein H, Siebert R, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016; 127: 2375-90.
- Strati P, Shanafelt TD. Monoclonal B-cell lymphocytosis and early-stage chronic lymphocytic leukemia: diagnosis, natural history, and risk stratification. Blood. 2015; 126: 454-62.
- Villegas-Gracia R, Jaramillo Arbeláez P, Cardona-Arias JA. Prevalencia de linfocitosis monoclonal de células b y factores asociados: metaanálisis 2002-2012. Rev Cuba HematolInmunolHemoter. junio de 2015; 31: 172-86.
- Kostopoulos IV, Paterakis G, Pavlidis D, Kastritis E, Terpos E, et al. Clonal evolution is a prognostic factor for the clinical progression of monoclonal B-cell lymphocytosis. Blood Cancer J. agosto de. 2017; 7: e597.
- Rodríguez‐Preciado SY, Magaña‐Torres MT, Cruz ARJ, Barros‐ Núñez P. Detection of monoclonal B cells in general population from two different regions of Mexico. Int J Immunogenet. 2017; 44: 279-85.
- de Faria-Moss M, Yamamoto M, Arrais-Rodrigues C, Criado I, Gomes CP, et al. High frequency of chronic lymphocytic leukemia-like low-count monoclonal B-cell lymphocytosis in Japanese descendants living in Brazil.Haematologica. junio de. 2020; 105: e298-301.
- Villegas Gracia R, Franco Alzate C, Rendón Henao J, Torres Hernández JD, Jaramillo Arbelaez PE. Frequency of monoclonal B-cell lymphocytosis in relatives of patients with chronic lymphocytic leukemia. Colomb Medica Cali Colomb. 2016; 47: 81-6.
- Van Dongen JJM, Lhermitte L, Böttcher S, Almeida J, van der Velden VHJ, et al. EuroFlow antibody panels for standardized n-dimensional flow cytometric immunophenotyping of normal, reactive and malignant leukocytes. Leukemia. 2012; 26: 1908-75.
- Nieto WG, Teodosio C, López A, Rodríguez-Caballero A, Romero A, et al. Non-CLL-like monoclonal B-cell lymphocytosis in the general population: prevalence and phenotypic/genetic characteristics. Cytometry B ClinCytom. 2010; 78: S24-34.
- Nieto WG, Almeida J, Romero A, Teodosio C, López A, et al. Increased frequency (12%) of circulating chronic lymphocytic leukemia–like B-cell clones in healthy subjects using a highly sensitive multicolor flow cytometry approach. Blood. 2009; 114: 33-7.
- Fogarty H, Dowling A, O’Brien D, Langabeer S, Bacon CL, Flavin R, et al. Biclonallymphoproliferative disorders: another association with NOTCH1-mutated chronic lymphocytic leukaemias. Ir J Med Sci. 2021; 190: 1087-94.
- Sanchez ML, Almeida J, Gonzalez D, Gonzalez M, Garcia-Marcos MA, et al. Incidence and clinicobiologic characteristics of leukemic B-cell chronic lymphoproliferative disorders with more than one B-cell clone. Blood. 2003; 102: 2994-3002.
- Criado I, Rodríguez-Caballero A, Gutiérrez ML, Pedreira CE, Alcoceba M, et al. Low-count monoclonal B-cell lymphocytosis persists after seven years of follow up and is associated with a poorer outcome. Haematologica. 2018; 103: 1198-208.
- Shanafelt TD, Kay NE, Parikh SA, Achenbach SJ, Lesnick CE, et al. Risk of serious infection among individuals with and without low count monoclonal B-cell lymphocytosis (MBL). Leukemia. 2020; 1-6.
- Scarfò L, Ghia P. What does it mean I have a monoclonal B-cell lymphocytosis?: Recent insights and new challenges. Semin Oncol. 2016; 43: 201-8.
- Hagner PR, Mazan-Mamczarz K, Dai B, Corl S, Zhao XF, Gartenhaus RB. Alcohol consumption and decreased risk of non-Hodgkin lymphoma: role of mTOR dysfunction. Blood. 2009; 113: 5526-35.
- Sergentanis TN, Ntanasis-Stathopoulos I, Tzanninis IG, Gavriatopoulou M, Sergentanis IN, et al. Meat, fish, dairy products and risk of hematological malignancies in adults – a systematic review and meta-analysis of prospective studies. LeukLymphoma. 2019; 60: 1978-90.
- Matos DM, Furtado FM, Falcão RP. Monoclonal B-cell lymphocytosis in individuals from sporadic (non-familial) chronic lymphocytic leukemia families persists over time, but does not progress to chronic B-cell lymphoproliferative diseases. Rev Bras Hematol E Hemoter. 2015; 37: 292-5.