
Research Article
Austin J Clin Med. 2016; 3(1): 1024.
Predictors of Relapse in Clostridium Difficile Associated Diarrhea
Rapose A* and Upadhyay J
Department of Medical Oncology, National Institute of Oncology, Morocco
*Corresponding author: Alwyn Rapose, Division of Infectious Diseases, Saint Vincent Hospital and Reliant Medical Group, 123 Summer Street, Suite 220, Worcester, USA
Received: January 05, 2016; Accepted: March 07, 2016; Published: March 09, 2016
Abstract
Background: Clostridium difficile infection (CDI) continues to manifest with increasing incidence and severity. High rates of relapses even in patients treated appropriately with metronidazole and / or oral vancomycin have led to increased hospital readmissions, morbidity and mortality. The search for a “cure” has resulted in research towards alternate therapies like fecal transplant, monoclonal antibodies, vaccines, and fidaxomicin. However, all the newer modalities carry significant cost compared to the antibiotics metronidazole and vancomycin. Establishing predictors for relapse would potentially help in targeted use of advanced therapies to a subset of patients with CDI who have a higher risk of relapse. Our study aimed to determine the clinical variables that may predict CDI relapse. We also revisited the role of fecal leukocytes in prediction of relapse.
Methods: A retrospective chart analysis of the patients hospitalized with diagnosis CDI over a one-year period was performed. Patients with first episode of CDI who had a 3-month follow-up after discharge were evaluated. Abstracted data included standard demographics, prior antibiotic and Proton Pump Inhibitor (PPI) use, laboratory data and fecal WBC at admission. Relapses of CDI were determined by review of electronic medical records.
Results: Charts of 104 patients were reviewed. 60 patients satisfied inclusion criteria. 14 (23%) had a relapse in the 3 months following discharge; 12 of 49 patients (25%) > 60 years of age relapsed compared to 2 out of 11 (18%) of those < 60; 10 of 25 (40%) with positive fecal leukocytes relapsed, while only 3 of 23 (13%) with negative fecal leukocytes relapsed. Of the patients with exposure to a single antibiotic course in the previous 6 months 20% experienced a relapse, while in patients who received two and more courses of antibiotics over the previous 6 months, there was 33% relapse rate. A combination of age > 60 years and positive fecal WBC was associated with a relapse rate of 42% (8 out of 19) as compared to no relapses in patients with a combination of age < 60 years and negative fecal WBC.
Conclusions: The combination of age > 60 years, fecal leukocyte positivity and exposure to multiple antibiotic courses prior to initial CDI predisposes patients to a relapse. These simple predictors may help physicians to target the newer advanced treatments to CDI patients with these risk factors.
Introduction
CDI is the leading cause of healthcare associated infections and may be the leading cause of death from gastroenteritis-type illness in the United States of America [1,2]. Every year CDI accounts for a large number of hospitalizations, is linked to almost 14,000 deaths, adding an estimated $897 million to $1.3 billion in annual healthcare costs [3]. It has become such a widespread problem that it was called a “major public health threat” at the Society for Healthcare Epidemiology of America (SHEA) at their 2011 annual scientific meeting.
Relapsing CDI presents additional challenges. Recent data indicates that the relapse rate has increased to 30% after first episode of CDI, 45% after first recurrence and up to 60% after 2 or more recurrences. Needless to say that relapsing CDI can be difficult to treat and leads to substantially increased morbidity and mortality. There is published literature and society statements regarding the management of relapsing CDI [4,5], some studies suggesting therapies like cyclical courses of antibiotics [6]. More recent publications have claimed improved results with newer approaches like fecal transplant, monoclonal antibodies, vaccines, and fidaxomicin. However, these modalities carry significant cost compared to the established antibiotics metronidazole and vancomycin. Therefore another important focus of research is to establish predictors for relapse which would potentially help in targeted use of advanced therapies to a subset of patients with CDI who have a higher risk of relapse.
Methods
We performed a retrospective chart review of patients admitted for CDI to Saint Vincent Hospital, a community hospital in Worcester, Massachusetts, between July 2008 and June 2009. All patients aged >18years and with first episode of CDI were evaluated. A case patient was defined as having diarrhea and positive stool enzyme immunoassay for C. difficile Toxin A/B (Meridian Bioscience Inc., Cincinnati, OH.) Extracted data included patient demographics, presentation from community v/s health-care facility, pre-hospitalization medications, antibiotics received within last 6 months, physical parameters, laboratory data, and length of hospital stay. Patients who were admitted with relapsed episode and patients who died during hospitalization or within a week of discharge were excluded. Followup records were reviewed for a relapse up to 3 months after discharge. Approval for the study was obtained from the hospital’s Institutional Review Board. We performed logistic regression on the different risk factors under study. Risk factor variables included age, exposure to single v/s multiple courses of antibiotics, presentation from home v/s from health-care facility, length of hospital stay, PPI use, WBC count and presence or absence of fecal leucocytes. We considered p values less than 0.05 to be significant.
Results
Study population/ Demographics
Charts of 104 patients admitted with CDI during the study period were reviewed. Out of these, 43 patients were excluded - including 23 who were admitted for a relapse episode and 20 patients who died during the hospitalization or within 1 week after discharge from the hospital. 61 patients fulfilled our inclusion criteria, one patient was lost to follow up and hence 60 patients formed our study population. 38% (n=23) were males and 63% (n=37) females. Twenty three percent of patients (14/60) had a relapse during the 3-month follow up period (Table 1). The majority of our patients were older, with 50% above the age of 80 years (n=30). 33% of patients above age 80 years (10/30) experienced a relapse, in contrast to 13% of those less than 80 (4/30) (p=0.049) ((Figure 1), panel 1). 10 out of 25 (40%) patients with positive fecal leukocytes relapsed, while only 3 out of 23 (13%) with negative fecal leukocytes relapsed (p=0.028) ((Figure 1), panel 2). 12 patients did not have test for fecal leucocytes. A combination of age > 60 years and positive fecal WBC was associated with a relapse rate of 42% (8 out of 19) as compared to 0 relapses in patients with a combination of age < 60 years and negative fecal WBC. ((Figure 1), panel 3). Other observations that did not reach statistical significance included 20% of the patients with exposure to a single antibiotic course in the previous 6 months experienced a relapse, while 33% of patients who received two and more courses of antibiotics over the previous 6 months relapsed. 36 patients presented from home, of which 8 (22%) experienced a relapse, 24 patients presented from a health-care facility of which 5 (20%) experienced a relapse. Out of 25 patients who had WBC > 15000 at admission 6 (24%) experienced a relapse, 35 patients had admission WBC <15000 of which 8 (22.8%) had a relapse. In terms of duration of hospital stay, 49 patients were discharged within 14 days, of these 12 (24.5%) patients experienced a relapse. 11 patients had an extended hospital course, of these 2 (18%) patients experienced a relapse.
No. of patients
Relapse (%)
Total
60
14 (23)
Age
<60
11
2 (18)
>60
49
12 (25)
Fecal WBC
positive
25
10 (40)
negative
23
3 (13)
Not done
12
1 (8)
Compound risk factors
Age>60 +
positive fecal WBC
19
8 (42)
Age<60 +
negative fecal WBC
4
0 (0)
Table 1: Results.
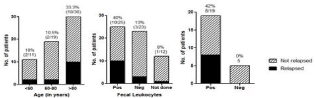
Figure 1: Comparing populations with and without relapse.
Regarding differences in relapse rates between patients on PPIs v/s not taking PPIs – of the 42 patients who were on a PPI at the time of CDI diagnosis, 8 (19%) developed a relapse, while out of the 18 patients not on a PPI, 6 (33%) developed a relapse.
Discussion
The relapse rate in our study was 23% consistent with national figure of relapse between 20-30%. Two factors - age greater than 80 years, and testing positive for fecal leucocytes on admission – were statistically significant differences between those who did and those who did not develop relapses. The combination of age < 60 years and negative fecal WBC resulted in no relapses in CDI. Other researchers have shown the increasing age, poor quality life scores, prolonged hospitalization or stay in a long term care facility, initial disease severity and continued use of non-C. difficile antibiotic after diagnosis of CDI was associated with an increase in the incidence of relapse [7-10].
In contrast to these reports, well-known predictors of mortality associated with CDI including peripheral WBC >15,000, community versus facility acquired CDI and duration of hospital stay did not show statistical relationship to the relapse rate in our study. Interestingly, use of PPIs was associated with decreased risk of recurrence (OR 0.18, p=0.06). This is in contrast to the common reported increased risk for relapse in patients on PPIs [11,12].
Our study also demonstrated considerable risk for relapse in those who had received more than one course of antibiotic in previous 6 months. This is consistent with studies by other authors [7-10]. Stevens et al have suggested that rather than the number of courses of individual antibiotics, it is the cumulative exposure to these antibiotics that is a potent risk factor for first and possibly subsequent episodes of CDI [13].
At the molecular level – various authors have pointed to different risk factors for relapse including infection with the B1NAP1/ 027 clone of C. difficile [14], specific colonic micro biota [15] and C. difficile surface layer proteins [16]. Unfortunately these tests are not readily available at most community hospitals.
Our data suggests that age > 80 years; fecal leukocyte positivity and exposure to multiple antibiotic courses prior to initial CDI predisposes patients to relapse. Fecal leukocytes, a relatively inexpensive and easily available test, may help serve as a predictor for relapse. Larger prospective studies are warranted to confirm the findings of our analysis. More recently – lymphopenia has been evaluated as a predictor for relapse [17]. This test is also routinely available and should add valuable information when the patient presents for the first time with CDI.
The targeted use of newer and expensive medications in these patients who have higher risks for relapse will decrease the overall health care cost associated with CDI.
References
- Magill SS, Edwards JR, Bamberg W, Jonathan R Edwards, M Stat, Zintars G Beldavs, et al. Multistate point-prevalence survey of health care-associated infections. Emerging Infections Program Healthcare-Associated Infections and Antimicrobial Use Prevalence Survey Team. N Engl J Med. 2014; 370: 1198-1208.
- Hall AJ, Curns AT, McDonald LC, Parashar UD, Lopman BA. The roles of Clostridium difficile and norovirus among gastroenteritis-associated deaths in the United States, 1999-2007. Clin Infect Dis. 2012; 55: 216-223.
- Dubberke ER, Reske KA, Olsen MA, McDonald LC, Fraser VJ. Short- and long-term attributable costs of Clostridium difficile-associated disease in nonsurgical inpatients. Clin Infect Dis. 2008; 46: 497-504.
- Pépin J, Routhier S, Gagnon S, Brazeau I. Management and outcomes of a first recurrence of Clostridium difficile-associated disease in Quebec, Canada. Clin Infect Dis. 2006; 42: 758-764.
- Cohen SH, Gerding DN, Johnson S, Kelly CP, Loo VG, McDonald LC. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the society for healthcare epidemiology of America (SHEA) and the infectious diseases society of America (IDSA). Infect Control Hosp Epidemiol. 2010; 31: 431-455.
- Johnson S, Schriever C, Galang M, Kelly CP, Gerding DN. Interruption of recurrent Clostridium difficile-associated diarrhea episodes by serial therapy with vancomycin and rifaximin. Clin Infect Dis. 2007; 44: 846-848.
- McFarland LV, Surawicz CM, Rubin M, Fekety R, Elmer GW, Greenberg RN. Recurrent Clostridium difficile disease: epidemiology and clinical characteristics. Infect Control Hosp Epidemiol. 1999; 20: 43-50.
- Pépin J, Saheb N, Coulombe MA, Alary ME, Corriveau MP, Authier S. Emergence of fluoroquinolones as the predominant risk factor for Clostridium difficile-associated diarrhea: a cohort study during an epidemic in Quebec. Clin Infect Dis. 2005; 41: 1254-1260.
- Eyre DW, Walker AS, Wyllie D, Dingle KE, Griffiths D, Finney J, et al. Predictors of first recurrence of Clostridium difficile infection: implications for initial management. Infections in Oxfordshire Research Database.Clin Infect Dis. 2012; 55: 77-87.
- Garey KW, Dao-Tran TK, Jiang ZD, Price MP, Gentry LO, Dupont HL. A clinical risk index for Clostridium difficile infection in hospitalised patients receiving broad-spectrum antibiotics. J Hosp Infect. 2008; 70: 142-147.
- McDonald EG, Milligan J, Frenette C, Lee TC. Continuous Proton Pump Inhibitor Therapy and the Associated Risk of Recurrent Clostridium difficile Infection. JAMA Intern Med. 2015; 175: 784-791.
- Zilberberg MD, Reske K, Olsen M, Yan Y, Dubberke ER. Risk factors for recurrent Clostridium difficile infection (CDI) hospitalization among hospitalized patients with an initial CDI episode: a retrospective cohort study. BMC Infect Dis. 2014; 14: 306.
- Stevens V, Dumyati G, Fine LS, Fisher SG, van Wijngaarden E. Cumulative antibiotic exposures over time and the risk of Clostridium difficile infection. Clin Infect Dis. 2011; 53: 42-48.
- Marsh JW, Arora R, Schlackman JL, Shutt KA, Curry SR, Harrison LH. Association of relapse of Clostridium difficile disease with BI/NAP1/027. J Clin Microbiol. 2012; 50: 4078-4082.
- Manges AR, Labbe A, Loo VG, Juli K Atherton, Marcel A Behr, Luke Masson, et al. Comparative metagenomic study of alterations to the intestinal microbiota and risk of nosocomial Clostridum difficile-associated disease. J Infect Dis. 2010; 202: 1877-1884.
- Collins LE, Lynch M, Marszalowska I, Kristek M, Rochfort K, O’Connell M. Surface layer proteins isolated from Clostridium difficile induce clearance responses in macrophages. Microbes Infect. 2014; 16: 391-400.
- Lavergne V, Beauséjour Y, Pichette G, Ghannoum M, Su SH. Lymphopenia as a novel marker of Clostridium difficile infection recurrence. J Infect. 2013; 66: 129-135.