
Special Article - Pulmonary Hypertension
Austin J Clin Med. 2016; 3(1): 1028.
Post-Partum Dyspnea in a Previously Asymptomatic 22 Year Old: Management of Rapidly Progressive Pulmonary Arterial Hypertension in the Acute and Post Acute Setting
Johnson AE¹, Rivera-Lebron BN², Risbano MG², Holtz JE², Teuteberg JJ², Ramani RN¹, Songdechakraiwut T³, Shah DK³, Hickey GW¹, Lagazzi LF³, Berlacher KL¹ and Simon MA¹*
¹Heart and Vascular Institute, University of Pittsburgh Medical Center, USA
²Department of Medicine, University of Pittsburgh, Pittsburgh, Pennsylvania, USA
³Department of Cardiothoracic Surgery, Division of Cardiac Surgery, UPMC Presbyterian, USA
*Corresponding author: Simon MA, Heart and Vascular Institute, University of Pittsburgh Medical Center, 200 Lothrop Street, Scaife Hall, Pittsburgh, USA
Received: September 21, 2016; Accepted: October 07, 2016; Published: October 10, 2016
Abstract
A 22-year-old woman was transferred from another hospital after developing precipitous postpartum hypoxemia and hypotension. Upon arrival, the patient was severely hypoxic with oxygen saturation under 20% despite mechanical ventilation. Physical examination was notable for signs of right heart failure. Progressive bradycardia and asystolic arrest ensued. Prolonged resuscitation, necessitated cannulation for veno-venous Extra Corporeal Membrane Oxygenation (ECMO). A patent foramen ovale with right to left shunt and elevated pulmonary pressures was identified by transesophageal echocardiogram. Right to left intracardiac shunting can be seen in chronic Pulmonary Arterial Hypertension (PAH), but does not fully explain the severe acute decompensation. Amniotic Fluid Embolism (AFE) causes acute decompensation after delivery necessitating advanced resuscitative efforts. ECMO can serve as supportive therapy in unstable patients with AFE and PAH. This case highlights supporting evidence for AFE in the unique setting of severe PAH, as well as its diagnosis and management. Medical therapy for PAH is also reviewed.
Keywords: Pulmonary arterial hypertension; Amniotic fluid embolism; Patent foramen ovale; Intracardiac shunt
Abbreviations
AFE: Amniotic Fluid Embolism; PAH: Pulmonary Arterial Hypertension; TTE: Transthoracic Echocardiogram; ECMO: Extracorporeal Membrane Oxygenation; PE: Pulmonary Embolism; PA: Pulmonary Artery; PAH: Pulmonary Arterial Hypertension; RV: Right Ventricle; RVH: Right Ventricular Hypertrophy; LV: Left Ventricular; PFO: Patent Foramen Ovale; PCWP: Pulmonary Capillary Wedge Pressure
Case Presentation
History of present illness
A 22-year-old woman was transferred to our quaternary care facility with severe hypoxemia after childbirth. She had been reportedly healthy prior to and during pregnancy but noted mild dyspnea when climbing stairs toward the end of the pregnancy. Approximately 7 hours after a routine, full term, spontaneous vaginal delivery, the patient developed acute onset of respiratory failure with refractory hypoxemia necessitating intubation. She was initiated on vasopressors for shock. A CT angiogram of the chest ruled out Pulmonary Embolism (PE), but revealed Pulmonary Artery (PA) enlargement, concerning for Pulmonary Arterial Hypertension (PAH). Due to persistent hypoxia, the patient was started on NO and transferred to our facility.
Additional medical history
The patient was gravida 1, para 1. She had a history of childhood asthma, endometriosis status post laparoscopic removal, and migraine headaches. Prior to hospitalization, she was taking prenatal vitamins and no other medications. Family history was notable for 2 uncles who died from unknown congenital heart disease. The patient smoked a half pack of cigarettes per day since age 15, but quit during pregnancy.
Physical examination
Vital signs on admission were: temperature 37 degrees, heart rate 141 beats per minute, blood pressure 79/46 mmHg, oxygen saturation 60%. Ventilator settings were rate of 22 breaths per minute, 100% FiO2, and PEEP of 15 cm of water. The patient was intubated and comatose. Pupillary response was normal. The neck was supple and the jugular venous pressure did not appear elevated. She was breathing at the rate of the ventilator. There were no adventitial lung sounds. There was a fixed split second heart sound, but no murmurs, rubs, or gallops. There was no sternal lift and the point of maximal impact was not displaced. The digits were mildly cyanotic, but there was no clubbing or edema.
Initial acute management
Severe hypoxia persisted and oxygen saturation deteriorated despite suctioning, ventilator recruitment maneuvers and bag-valve. A bronchoscopy excluded the presence of obstructive mucous plugs. The heart rate became progressively bradycardic. She then developed pulseless electrical activity and asystole. Resuscitative measures included chest compressions and epinephrine bolus then continuous infusion. The patient regained spontaneous circulation briefly then again became bradycardic and pulseless.
Upon resumption of chest compressions, the patient’s oxygen saturation would improve to the 40-50% range. However spontaneous circulation and oxygen saturations were short-lived as the patient repeatedly required CPR. The patient was placed on vasopressor support with epinephrine, norepinephrine, and vasopressin infusions.
A bedside Transthoracic Echocardiogram (TTE) revealed massively dilated Right Ventricle (RV) with severe diffuse hypokinesis and Right Ventricular Hypertrophy (RVH). The right atrium was dilated. There was malcoaptation of the visualized tricuspid leaflets. Left ventricular (LV) systolic function was mildly decreased.
Due to severe hypoxia, veno-venous ECMO was initiated with flows of 5L. Oxygen saturation improved to 90%.
Laboratory data and studies
Arterial blood gas on arrival: pH 7.21, PaCO2 49mmHg, PaO2 <30mmHg, HCO3 19mEq/L, base deficit 8mEq/L, SO2 18%. Troponin was only mildly elevated at 0.68. A basic metabolic panel was normal. Lactate was 3.7 and increased to 12.3 minutes later. Complete blood count was significant for leukocytosis of 17, 900cells/uL, hemoglobin 9.2g/dL and platelets 82,000/uL. INR was 2.0. Bronchoalveolar lavage 4 days after admission showed many white blood cells but normal respiratory flora.
Electrocardiogram upon arrival revealed sinus tachycardia, right atrial abnormality, right axis deviation, and incomplete right bundle branch block. Chest x-ray showed diffuse hazy lung opacities. Transesophageal echocardiogram performed the day after admission revealed normal LV size and thickness. LV systolic function was mildly hypokinetic with an ejection fraction of 45%. There was flattening of the intraventricular septum consistent with RV pressure overload (Figure 1). RV function was severely decreased with RVH. The right atrium was giant. There was a persistent Eustachian valve (Figure 2) and a patent foramen ovale (0.7cm diameter) with a right to left shunt (Figure 3). The main PA was severely dilated at 3.8cm with echodensity suspicious for embolic material. PA catheter confirmed a pressure of 97/59mmHg.
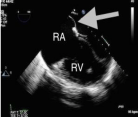
Figure 1: Transesophageal echocardiogram image showing flattening of
the intraventricular septum consistent with Right Ventricle (RV) pressure
overload. The RV is thickened consistent with RVH. The Right Atrium (RA)
is massively dilated with bowing of the intra-arterial septum toward the left
atrium (arrow).
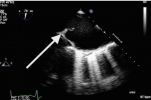
Figure 2: Prominent Eustachian valve (arrow).
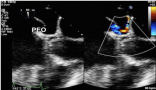
Figure 3: Patent Foramen Ovale (PFO) with color flow Doppler demonstrating
a right to left shunt.
Case Synthesis
Fixed splitting of the second heart sound and dilated, hypertrophied RV with relatively preserved LV function were important findings prompting early PAH therapy. Conceivably, the patient’s RV function worsened during pregnancy, but this does not explain acute hypoxia post delivery. The differential diagnosis of her acute decompensation includes primary cardiac, pulmonary, and vascular etiologies. Infection was unlikely. PE was ruled out with computed tomography. Peripartum cardiomyopathy can cause fulminant heart failure, however the patient’s LV systolic function was relatively preserved. Intravascular volume shifts occur with pregnancy leading to increased RA and RV pressure, especially for patients with PAH [1]. Women with PAH are at risk for decompensation between weeks 20-24, but acute decompensation hours after delivery is less common [2,3]. Alternatively, the acute presentation was secondary to Amniotic Fluid Embolism (AFE). AFE is a clinical diagnosis [4] requiring the presence of certain findings (Table 1) and exclusion of other diagnoses. Hypotension, cyanosis, hypoxia, hazy infiltrates on chest x-ray, altered mental status, and coagulopathy and are often concomitant (Table 1) [5]. All of these findings were present in our patient. Although we did not have tissue diagnosis of fetal material in the pulmonary vasculature, presence of echogenic material in the PA is suggestive.
Obstetric: Intrauterine pregnancy*, tumultuous childbirth.
Cardiovascular: tachycardia*, precipitous hypotension*, right ventricular failure*, cardiac arrest*
Pulmonary: Respiratory distress*, tachypnea*, dyspnea, rapid onset hypoxia*, hazy bilateral infiltrates on chest imaging*
Hematologic: Coagulopathy (elevated INR*, aPTT, PT, or fibrinogen), disseminated intravascular coagulation (DIC), thrombosis*
Neurologic: Seizures, confusion, agitation.
Supportive Pathologic Findings: Presence of fetal material in the pulmonary vasculature, elevated tryptase or histamine levels, decreased C3 and C4 complement levels.
Adapted from Kaur, et al. [10].
*Findings present in our patient.
Table 1: Findings Associated with Amniotic Fluid Embolism.
Continued hospital course
The patient was maintained on ECMO flows up to 5.9L, yet PA pressures remained elevated (Table 2). Inhaled NO was restarted after initial discontinuation on admission, but hypoxia persisted. Initial attempts with IV pulmonary vasodilators were unsuccessful at reducing pulmonary pressures and caused systemic hypotension. We briefly added inhaled epoprostenol, which has been shown to be effective in critically ill hypoxic patients [6-8]. Due to the refractory nature of her course, the lung and heart transplant teams discussed the patient’s candidacy for potential lung and/or heart transplant. The patient slowly began to improve and ventilator settings were weaned. She remained on ECMO for a total of 5 weeks. Finally, oxygenation and PA pressures began to improve with continued up titration of IV epoprostenol with ECMO decannulation on day 35. Ultimately transplant was deferred in favor of medical management. PFO closure was discussed but deferred in the acute setting. Neurologically, the patient returned to baseline.
Post acute care management
Since her hospitalization, she underwent surgery under general anesthesia for foot gangrene secondary to thrombosis while on ECMO. PA pressures were measured intraoperatively and only mildly elevated per anesthesia records. The patient’s medication regimen had included trepostinil since hospital discharge, which was changed to ambrisentan in light of the reassuring intraoprative PA pressures (Table 2). She has not required diuretic therapy as an outpatient and reported minimal functional limitation.
Hospital Day
Medication Regimen
RA Pressure
PA Pressure
Other Clinic Findings
2
Epinephrine *
Norepinephrine *
Vasopressin *
Inhaled Epo 720mcg, q4h
85/65
Patient remains intubated in critical condition
3
Epinephrine *
Norepinephrine *
Vasopressin *
Inhaled Epo 720mcg, q4h
Inhaled NO 20 ppm x1 dose
75/56
Requiring high ventilator settings to maintain oxygenation
4
Epinephrine *
Norepinephrine *
Vasopressin *
Inhaled Epo 720mcg, q4h
IV Epo 2ng/kg/min
79/60
Fluid retention unresponsive to diuresis, dialysis initiated
5
Norepinephrine *
IV Epo 4ng/kg/min
19
101/51
Initial hypotension upon epoprostenol initiation
PFO closure aborted
37
IV Epo 30ng/kg/min
Sildenafil 10mg
55/30
ECMO decannulation
60
Inhaled Treprostinil 9 puffs q6h
Sildenafil 20mg
Furosemide 20mg
Day of discharge
1.5 years after d/c
Ambrisentan 10mg
Sildenafil 20mg
3
70/30
Patient having dyspnea with moderate exertion
NO = Nitric oxide.
d/c = Discharge.
Epo = Epoprostenol.
*Continuous infusion, titrated by nursing protocol.
Pressures are in mmHg. Medication frequencies are daily unless otherwise noted.
Table 2: Right heart catheterization and pulmonary artery catheter results as a response to therapy.
Five months after hospital discharge, an outpatient TTE revealed persistent PAH and dilated RV but normal function. The PFO was redemonstrated with just scant right to left intracardiac shunt by saline contrast. Ultimately, the patient was deemed not to be a candidate for PFO closure after stabilization on medical therapy.
A year and a half after her index presentation, repeat right heart catheterization showed moderate-severe PAH (Table 2). Pulmonary vascular resistance was 8 Woods units using thermodilution calculation of cardiac output. A ventilation/perfusion scan was low probability for chronic PE as a potential contributor to her PAH and argues against venous thromboembolism as the cause of hypoxic arrest on initial presentation. She is now being initiated on selexipag.
Discussion and Conclusions
This is a 22-year-old woman with exertional dyspnea toward the end of her pregnancy who developed cardiopulmonary collapse after a vaginal delivery and was found to have a PFO with PAH. Though more commonly seen with atrial or ventricular septal defects, over 14% of intracardiac shunt related- PAH cases are among patients with PFO [9]. Right-to-left shunt was likely exacerbated by the persistence of the Eustachian valve. Women with PAH are at high risk for decompensation during pregnancy [2,3]. However, the acuity of this patient’s decompensation hours after delivery was likely triggered by an insult such as AFE. Usually attempts at AFE diagnosis are retrospective after acute management [10,11]. Some suggest a biphasic response in AFE: first RV failure/PAH, followed by LV failure [12]. Our patient was on veno-venous ECMO, which allowed for adequate oxygenation in the setting of severe intracardiac shunting. Case reports document use of pulmonary vasodilators such as NO [13] and resuscitative measures like ECMO [14] in refractory AFE cases. There have not been published cases of patients with chronic PAH and acute AFE.
This case also highlights the medical options for patients with PAH. Initial therapy included NO, a pulmonary vasodilator used in the acute critically ill setting, as the patient’s care was transitioned from the outside hospital. Next, we used intravenous epoprostenol, a prostacyclin, as dose can be rapidly increased in the acute setting. A moderate dose of 30ng/kg/min allowed eventual ECMO weaning. Notably, up titration can be limited by systemic hypotension and symptoms such as headache, flushing, and diarrhea, all of which should be aggressively treated so as not to prevent achievement of an effective dose. After stabilization on epoprostenol, we successfully transitioned to inhaled treprostinil, a prostacyclin, and sildenafil, an oral phosphodiesterase type 5 inhibitor. We favored these medications over IV epoprostenol for ease of administration given the patient’s remote rural community, and the rapid functional recovery once weaned from ECMO. Treprostinil eventually was discontinued in favor of an oral regimen of sildenafil and ambrisentan, an endothelin receptor antagonist, after demonstrating several months of stability. Selexipag is an oral selective intrapulmonary prostacyclin-receptor agonist and will be added as a third-line agent to treat PAH with mild functional limitation.
References
- Svetlichnaya J, Janmohammed M, De Marco T. Special Situations in Pulmonary Hypertension: Pregnancy and Right Ventricular Failure. Cardiology clinics. 2016; 34: 473-487.
- Bedard E, Dimopoulos K, Gatzoulis MA. Has there been any progress made on pregnancy outcomes among women with pulmonary arterial hypertension? European heart journal. 2009; 30: 256-265.
- Galie N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). European heart journal. 2016; 37: 67-119.
- Pacheco LD, Saade G, Hankins GDV, Clark SL. Amniotic fluid embolism: diagnosis and management. American Journal of Obstetrics & Gynecology. 2016; 215: 16-24.
- Gist RS, Stafford IP, Leibowitz AB, Beilin Y. Amniotic fluid embolism. Anesthesia and analgesia 2009; 108: 1599-1602.
- Khan TA, Schnickel G, Ross D, Bastani S, Laks H, Esmailian F, et al. A prospective, randomized, crossover pilot study of inhaled nitric oxide versus inhaled prostacyclin in heart transplant and lung transplant recipients. The Journal of thoracic and cardiovascular surgery. 2009; 138: 1417-1424.
- McGinn K, Reichert M. A Comparison of Inhaled Nitric Oxide Versus Inhaled Epoprostenol for Acute Pulmonary Hypertension Following Cardiac Surgery. The Annals of pharmacotherapy. 2016; 50: 22-26.
- Torbic H, Szumita PM, Anger KE, Nuccio P, LaGambina S, Weinhouse G. Inhaled epoprostenol vs inhaled nitric oxide for refractory hypoxemia in critically ill patients. Journal of critical care. 2013; 28: 844-848.
- Saha A, Balakrishnan KG, Jaiswal PK, Venkitachalam CG, Tharakan J, Titus T, et al. Prognosis for patients with Eisenmenger syndrome of various aetiology. International journal of cardiology. 1994; 45: 199-207.
- Kaur K, Bhardwaj M, Kumar P, Singhal S, Singh T, Hooda S. Amniotic fluid embolism. Journal of anaesthesiology, clinical pharmacology. 2016; 32: 153-159.
- Kumar V, Khatwani M, Aneja S, Kapur KK. Paradoxical amniotic fluid embolism presenting before caesarean section in a woman with an atrial septal defect. International journal of obstetric anesthesia. 2010; 19: 94-98.
- Clark SL. New concepts of amniotic fluid embolism: a review. Obstetrical & gynecological survey 1990; 45: 360-368.
- McDonnell NJ, Chan BO, Frengley RW. Rapid reversal of critical haemodynamic compromise with nitric oxide in a parturient with amniotic fluid embolism. International journal of obstetric anesthesia. 2007; 16: 269-273.
- Hsieh YY, Chang CC, Li PC, Tsai HD, Tsai CH. Successful application of extracorporeal membrane oxygenation and intra-aortic balloon counterpulsation as lifesaving therapy for a patient with amniotic fluid embolism. American journal of obstetrics and gynecology. 2000; 183: 496-497.
Citation: Johnson AE, Rivera-Lebron BN, Risbano MG, Holtz JE, Teuteberg JJ, Ramani RN, et al. Post-Partum Dyspnea in a Previously Asymptomatic 22 Year Old: Management of Rapidly Progressive Pulmonary Arterial Hypertension in the Acute and Post Acute Setting. Austin J Clin Med. 2016; 3(1): 1028. ISSN:2381-9146