
Research Article
Austin J Clin Med. 2016; 3(1): 1029.
External Counterpulsation Therapy for Patients with Mild Cognitive Impairment
Moriarty PM1*, Badawi AH1, Lepping RJ2, Nolte B1, Tye SL1, Burns JM3, Bishara M4, Dutton JA1, Sehar N1, Denney LK1, Tennant HM1 and Brooks WB2
1Division of Clinical Pharmacology, The University of Kansas Medical Center, Kansas City, KS, USA
2Hoglund Brain Imaging Center, The University of Kansas Medical Center, Kansas City, KS, USA
3Alzheimer’s Disease Center, The University of Kansas Medical Center, Kansas City, KS, USA
4Department of Ophthalmology, The University of Kansas Medical Center, Kansas City, KS, USA
*Corresponding author: Moriarty PM, Division of Clinical Pharmacology, University of Kansas Medical Center, 3901 Rainbow Blvd, Mail Stop 3008, Kansas City, USA
Received: September 16, 2016; Accepted: October 07, 2016; Published: October 10, 2016
Abstract
The development of vascular dementia and Alzheimer’s Disease (AD) share an association with hemodynamic risk factors. Physical exercise improves cardiac output, which in turn increases Cerebral Blood Flow (CBF) and may improve cognitive function. Unfortunately, patients with signs of dementia are not always able to exercise, due to cognitive or physical decline. The Enhanced External Counterpulsation (EECP) therapy system is a non-invasive device used for patients with chronic angina and mild heart failure. This study evaluated the CBF changes in patients (n=4) with Mild Cognitive Impairment (MCI) after 35 one hour sessions of EECP therapy over 7 weeks. EECP therapy improves CBF in certain brain regions and there is evidence that these effects may persist for 6 months post-treatment. Cognitive function appears to be correlated with CBF rate in the hippocampus and precuneus. Although this study is small, its results are promising and support the need for a larger randomized controlled trial.
Keywords: External Counterpulsation; Mild cognitive impairment; Dementia; Alzheimer’s disease; Cerebral blood flow
Abbreviations
AD: Alzheimer’s Disease; CBF: Cerebral Blood Flow; EECP: Enhanced External Counterpulsation; MCI: Mild Cognitive Impairment; CHF: Congestive Heart Failure; MRI: Magnetic Resonance Imaging; CDR: Clinical Dementia Rating; ADAS-cog: AD Assessment Scale-Cognitive Subscale; ROI: Regions of Interest
Introduction
There is a strong correlation between Alzheimer’s disease (AD) and poor Cerebral Blood Flow (CBF) [1]. Cognitive decline occurs naturally with age, however a more severe decline, as in Mild Cognitive Impairment (MCI), is a precursors to AD. Observational studies have shown that the treatment of vascular risk factors including: hypertension, diabetes, cerebrovascular diseases, and hypercholesterolemia, decrease the risk of dementia progression [2,3]. Physical exercise is the most basic and efficient way to improve blood flow [4]. Physical exercise has been shown to increase cardiac output, which in turn leads to an increase in CBF [5]. Wang et al and Varghese et al. have shown that elderly patients who partake in physical activity are less likely to develop dementia and experience cognitive decline [6,7]. Due to cognitive or physical decline, patients with signs of dementia are not always able to exercise. The Enhanced External Counterpulsation (EECP) therapy system is a non-invasive device. Traditionally, EECP is used to treat patients with chronic angina and Congestive Heart Failure (CHF) by increasing cardiac output and reducing the workload of the heart. EECP increases cardiac output by improving systolic unloading and venous return [8]. Patients with CHF have an increased risk of cognitive dysfunction presumably because of decreased CBF [9]. In a study done by Kozdag et al. EECP therapy resulted in improvement in all cognitive domains except visual and verbal memory tests in patients with CHF [9]. Thus, EECP may be used as an exercise alternative to increase CBF. The effects of EECP may act similarly to exercise therapy and are explored for patients with MCI in this study. Our objective was to evaluate CBF changes as well as changes in cognition in patients with MCI and no CHF or angina after 35 one hour sessions of EECP therapy. This was performed on a small sample for safety and feasibility in a cognitively impaired population. Future and larger outcome trials may be modeled after this small study.
Methods
Four patients, ages 64-74, were screened and selected from the University of Kansas Medical Center, with Clinical Dementia Rating (CDR) scores of 0.5, indicating MCI. Each study patient gave informed consent and understood the study rationale.
Patients received standard EECP (Figure 1, Vasomedical, Westbury, NY) therapy, consisting of 35 one hour treatments over a seven week period (5 treatments per week). The EECP machine contains 3 paired pressure cuffs wrapped around the calves and the lower and upper thighs. These cuffs are inflated sequentially (applying 250-300 mmHg of external pressure) starting from the calves during diastole and rapidly deflated during systole. The compressions and relaxations of the cuffs are paired with the R wave of the heart during the cardiac cycle. The cardiac cycles is monitored with a microprocessor that coordinate the inflation and deflation of the cuff with each heart beat via electrocardiogram interpretation.
Figure 1: EECP Machine. Vasomedical, Westbury, NY.
The Hachiniski Ischemic Score is used for differentiating types of dementia, patients with scores above 7 are indicative of vascular dementia and was used to exclude patients with severe vascular dementia in this study (mean= 3.5). Cognitive function of each patient was evaluated pre and post treatments using the AD Assessment Scale-Cognitive Subscale (ADAS-cog). Using a Siemens Skyra 3 Tesla scanner, arterial spin labeling perfusion Magnetic Resonance Imaging (MRI) measured CBF in three predetermined Regions of Interest (ROI); the hippocampus, inferior parietal lobe, and precuneus. Cognitive function and CBF were measured prior to EECP therapy, 24 hours after the last treatment, and 6 months post- EECP therapy. CBF of 20 healthy controls (CDR = 0) was measured with perfusion MRI for baseline comparison.
Results
In (Table 1), pre-EECP therapy, the MCI group had significantly lower CBF compared to cognitively normal (CDR =0) age-matched controls (n=20, Mann-Whitney U test). On average, brain CBF increased between 2.5-15.8% following EECP treatment (paired t-test; Table 1). The hippocampus saw the highest percent increase at 15.8%, the precuneus region showed a 13.6% increase and the inferior parietal lobe saw the smallest increase at 2.5%. In this small sample (n=4), CBF increase following EECP therapy approached statistical significance in the hippocampus (p=0.064) and precuneus (p=0.057). Moreover, at the six month follow up scan, there was some evidence that previously increased CBF was maintained (hippocampus) or possibly increased in some brain regions (precuneus).
Perfusion
(control)
(Mann-Whitney p)
Perfusion
(pre)
Perfusion
(post)
% increase
p-value
Perfusion
(Follow-up)
Hippocampus
37.8 (p=.007)
22.3
25.8
15.8%
0.064
24.5
Inferior parietal
lobe
28.6 (p=.001)
12.8
13.1
2.5%
0.216
12.9
Precuneus
37.4 (p=.013)
19.5
22.1
13.6%
0.057
24.4
Table 1: CBF group averaged data. On average, CBF increased between 2.5 – 15.8% after EECP therapy. In this small sample, this increase approached statistical significance in hippocampus and precuneus. Mean CBF in each region remained lower than healthy controls (p<0.05).
We also carried out voxel-wise paired t-tests to identify the voxels that were contributing to the ROI analyses above. The hippocampal and precuneal voxels displayed significantly higher flow after EECP therapy (p<0.05).
While this pilot study was underpowered to detect changes in the ADAS-cog scores with treatment, scores on this battery were correlated with changes in CBF specifically in the hippocampus. Three subjects received both baseline and post-EECP ADAS-cog score measurements showed improved hippocampal CBF that correlated with improved (i.e. lowered) dementia score (r²=0.78, Figure 2, 3). These results should be treated with caution since the sample was small and since we would expect dementia scores to worsen (i.e., increase) over time. Accordingly, even a lowering of the rate of decline could be interpreted as a positive outcome.
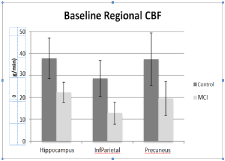
Figure 2: Model patient receiving treatment on EECP machine. Vasomedical
Inc, Westbury, NY.
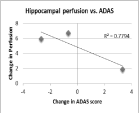
Figure 3: Change in hippocampal CBF compared to ADAS-cog score post-
EECP therapy. Improved hippocampal CBF is associated with decreases in
ADAS-cog score (r²=0.7794).
Discussion
We found that 35 hours of EECP therapy over 7 weeks improved CBF in patients with MCI compared to cognitively normal patients. Specifically, CBF in the hippocampus and precuneus regions improved following EECP therapy. These regions and the inferior parietal were of interest because of their involvement in cognition and cognitive decline [10].
Reduced CBF is strongly associated with the development of AD [1,2,10]. The vascular hypothesis of AD attributes disease development to age and vascular risks that leads to brain hypoperfusion [10]. Additionally, it is thought that increasing CBF contributes to increases in various neuroprotective mechanisms (angiogenesis, neurogenesis, synaptogenesis, and neurotransmitter synthesis) in cerebral areas involved in cognition and mobility [11]. In addition to these neuroprotective mechanisms, exercise is known to be one of the most potent non-pharmacological vascular protective interventions [12]. Improved CBF is thought to be caused by decreases in vascular oxidative stress and a reduction in the vascular burden [12]. The reduction in vascular burden indirectly reduces AD risk by improving vascular nitric oxide metabolism, vascular remodeling and regeneration, and endothelial signaling [13,14].
Exercise is not always a practical or possible option for an aging and cognitively declining population. This is where the EECP system comes into play; the EECP machine works in tandem with the circulatory system to reduce the work load of the heart and to improve cardiac output in patients with compromised cardiovascular systems. When used to treat patients with chronic angina and CHF, EECP therapy significantly improved quality of life and peripheral vascular function and functional capacity in coronary artery disease patients with ischemic left ventricle dysfunction to a similar degree to that seen in patients with coronary artery disease and preserved left ventricle function [15,16]. EECP therapy has also been found to increase cardiac output during treatment by 75% and reduce systemic vascular resistance by 20-30% [17]. EECP is not meant to replace exercise, its purpose in this study is to provide an alternative route to increase CBF.
It has been debated on whether EECP therapy has any benefit to CBF in the brain. Some think that the brain’s strict regulation of CBF within the blood brain barrier is constant and unchanging [18-20]. However, these data were obtained after a single treatment of EECP therapy and on patients without AD. We do know that AD patients show compromises in cerebrovascular structure [13] but is not known if the auto regulation of CBF is disrupted because of this. Yang and Wu proposed that EECP induces endothelial shear stress that may promote angiogenesis and vasculogenesis [14]. This proposed mechanism could affect CBF. Despite skepticism and an unclear mechanism, we were able to show that it is possible to increase and maintain CBF with EECP therapy in patients with MCI.
Our study was conducted as safety and feasibility pilot study for future use of EECP therapy in a cognitively declining population not for the purpose of finding statistically significant data. However, some significant and nearly significant data were found. Baseline regional CBF measurements showed that the MCI group had significantly lower CBF as compared to controls (p<0.05) in all 3 ROI. This baseline assessment justifies our interest in improving CBF in MCI patients and allowed us to measure improvements from EECP therapy.
Percent increase in MCI patients’ CBF approached significance in MCI patients’ hippocampal and precuneus regions. Additionally, 3 of the 4 subjects returned for post-EECP cognitive score measurements (ADAS-cog). The ADAS-cog is a brief cognitive battery aimed at measuring dementia severity. Lower composite scores on this battery indicate lower cognitive impairment. Scores indicated a correlation between increased hippocampal CBF and improved (i.e. lowered) dementia score. This finding provides further support for our hypothesis that EECP therapy may increase cognitive functioning in AD patients by increasing CBF in critical areas of the brain. Our study was underpowered to see an overall significant improvement on the ADAS-cog.
Likely with an increased sample size, CBF improvements would be significant following EECP therapy. Additionally, our study was underpowered to detect an overall improvement in ADAS-cog. Our results demand larger trials in order to determine if EECP therapy is an effective preventive method for cognitive decline.
EECP System Therapy improves CBF in certain brain regions and there is evidence that these effects may persist for 6 months post-treatment [21]. Cognitive function appears to be correlated with CBF rate in the hippocampus and precuneus. EECP Therapy System offers a non-invasive therapy option for patients with cognitive impairments without requiring the risk of physical activity. Although this study is small, its results are promising and support the need for a larger randomized controlled trial.
Acknowledgements
The investigators thank Vasomedical for donating an EECP machine to the University of Kansas, all staff involved in this study, and the patients and family members for giving their time.
References
- Sun ZW, Zhu YX, Liu HY, Liu J, Zhu XQ, Zhou JN, et al. Decreased cerebral blood flow velocity in apolipoprotein E epsilon4 allele carriers with mild cognitive impairment. European journal of neurology : the official journal of the European Federation of Neurological Societies. 2007; 14: 150-155.
- Li J, Wang YJ, Zhang M, Xu ZQ, Gao CY, Fang CQ, et al. Vascular risk factors promote conversion from mild cognitive impairment to Alzheimer disease. Neurology. 2011.
- Daulatzai MA. Cerebral hypoperfusion and glucose hypometabolism: Key pathophysiological modulators promote neurodegeneration, cognitive impairment, and Alzheimer's disease. Journal of neuroscience research. 2016.
- Erickson KI, Voss MW, Prakash RS, Basak C, Szabo A, Chaddock L, et al. Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci U S A. 2011; 108: 3017-3022.
- Paillard T. Preventive effects of regular physical exercise against cognitive decline and the risk of dementia with age advancement. Sports medicine - open. 2015; 1: 4.
- Wang HX KA, Winblad B, Fratiglioni L. Late-life engagement in social and leisure activities is associated with a decreased risk of dementia: a longitudinal study from the Kungsholmen project. American journal of epidemiology. 2002; 155: 1081-1087.
- Verghese JLR, Katz MJ, Hall CB, Derby CA, Kuslansky G, Ambrose AF, et al. Leisure activities and the risk of dementia in the elderly. N Engl J Med. 2003; 348: 2508-2516.
- Barsness G. Enhanced external counterpulsation in unrevascularizable patients. Curr Interv Cardiol Rep. 2001; 3: 37-43.
- Kozdag G, Iseri P, Gokce G, Ertas G, Aygun F, Kutlu A, et al. Treatment with enhanced external counterpulsation improves cognitive functions in chronic heart failure patients. Turk Kardiyoloji Dernegi arsivi : Turk Kardiyoloji Derneginin yayin organidir. 2013; 41: 418-428.
- Kim J, Kim YH, Lee JH. Hippocampus-precuneus functional connectivity as an early sign of Alzheimer's disease: a preliminary study using structural and functional magnetic resonance imaging data. Brain research. 2013; 1495: 18-29.
- Swain RA, Harris AB, Wiener EC, Dutka MV, Morris HD, Theien BE, et al. Prolonged exercise induces angiogenesis and increases cerebral blood volume in primary motor cortex of the rat. Neuroscience. 2003;117: 1037-1046.
- Paillard T, Rolland Y, de Souto Barreto P. Protective Effects of Physical Exercise in Alzheimer's Disease and Parkinson's Disease: A Narrative Review. Journal of clinical neurology (Seoul, Korea). 2015; 11: 212-219.
- Farkas E, Luiten PG. Cerebral microvascular pathology in aging and Alzheimer's disease. Progress in neurobiology. 2001; 64: 575-611.
- Yang DY, Wu GF. Vasculoprotective properties of enhanced external counterpulsation for coronary artery disease: beyond the hemodynamics. Int J Cardiol. 2013; 166: 38-43.
- Beck DT, Martin JS, Casey DP, Avery JC, Sardina PD, Braith RW. Enhanced external counterpulsation improves endothelial function and exercise capacity in patients with ischaemic left ventricular dysfunction. Clinical and experimental pharmacology & physiology. 2014; 41: 628-636.
- Tarpgaard Jorgensen M, May O. [Improvement of angina, quality of life, and working capacity after enhanced external counterpulsation]. Ugeskrift for laeger. 2013; 175: 114-116.
- Strobeck JE. Enhanced External Counterpulsation in Congestive Heart Failure: Possibly the Most Potent Inodilator to Date. Congestive Heart Failure. 2002; 8: 201-203.
- Marthol H, Werner D, Brown CM, Hecht M, Daniel WG, Hilz MJ. Enhanced external counterpulsation does not compromise cerebral autoregulation. Acta Neurol Scand. 2005; 111: 34-41.
- Jungehuelsing GJ, Liman TG, Brunecker P, Ebel A, Endres M, Buschmann I, et al. Does external counterpulsation augment mean cerebral blood flow in the healthy brain? Effects of external counterpulsation on middle cerebral artery flow velocity and cerebrovascular regulatory response in healthy subjects. Cerebrovasc Dis. 2010; 30: 612-617.
- Werner D, Marthol H, Brown CM, Daniel WG, Hilz MJ. Changes of cerebral blood flow velocities during enhanced external counterpulsation. Acta Neurol Scand. 2003; 107: 405-411.
- Bozorgi A, Mehrabi Nasab E, Sardari A, Nejatian M, Nasirpour S, Sadeghi S. Effect of Enhanced External Counterpulsation (EECP) on Exercise Time Duration and Functional Capacity in Patients with Refractory Angina Pectoris. The journal of Tehran Heart Center. 2014; 9: 33-37.