
Case Presentation
Austin J Clin Med. 2019; 6(1): 1039.
Successful Endovascular Treatment Strategy in an Unstable Patient with Complete Brachial Artery Transection and Thrombus at the Elbow Joint
Chen Z1, Zhong S2, Feng L3, Li M1,4* and Xi-quan Z1*
1Department of Interventional Vascular, 960 Hospital of PLA, Zibo, Shandong Province, China
2Department of Medical Imaging, Weifang Yidu Central Hospital, Weifang, Shandong, China
3Department of Medical Imaging, Zibo Maternal and Child Health Hospital, Zibo, Shandong, China
4Department of Medical Imaging, 960 Hospital of PLA, Jinan, Shandong Province, China
*Corresponding author: Min Li, Department of Medical Imaging, 960 Hospital of PLA, Jinan, Shandong Province, China
Xi-quan Zhang, M.D. Department of Medical Imaging, 960 Hospital of PLA, Jinan, Shandong Province, China
Received: April 08, 2019; Accepted: May 15, 2019; Published: May 22, 2019
Abstract
A 52-year-old patient presenting with right brachial artery transection at the elbow joint underwent emergent endovascular treatment. Upon arrival, he was hemodynamically unstable with hypotension and tachycardia. Selective angiography of the right subclavian artery was performed to confirm the injury site. We found a complete brachial artery transection at the elbow joint. The proximal end of the injured artery was blocked by a thrombus. The hemodynamical instability strongly suggested an emergent endovascular therapy to avoid a delay related to open-surgery repair. To avoid the coverage of branch vessels, a bare-metal stent was implanted with the radial-femoral working wire technique to repair the injured brachial artery. Then, the catheter thrombectomy was performed. The angiography demonstrated completely restored blood flow without contrast extravasation. No significant stenosis or stent fracture was observed during the 5-year follow-up period. A endovascular treatment strategy incorporating stent implantation and embolectomy can provide a suitable alternative to surgical repair to treat severed vascular injury complicated by a thrombus.
Keywords: Wounds and Injuries; Radiography; Interventional; Brachial Artery; Endovascular Procedures; Stent
Introduction
Although upper-extremity arterial injuries are relatively uncommon, they may lead to serious trauma outcomes. Delayed treatment for patients with arterial injury could increase the possibility of amputation.1 Endovascular repair is widely applied to patients who sustained vascular injuries due to a less invasive alternative with less blood loss [2]. Here, we present a case of complete right brachial artery transection at the elbow joint secondary to a crush injury. The proximal end of the injured artery was blocked by a thrombus. We successfully performed a successful “one-stop” endovascular treatment to repair the injured brachial artery and remove the thrombus in one session. A 5-year follow-up demonstrated that the endovascular treatment strategy may be an efficient means for artery transection and associated thrombus at the elbow joint.
Case Presentation
A 52-year-old man who had clinical suspicion of a right upperextremity arterial injury was referred to our hospital because of a blunt injury. When he arrived at the local hospital’s emergency department, he presented with pain, swelling and extensive soft tissue injury at the right elbow joint and forearm. His blood pressure was 140/95mmHg, and his heart rate was 78 beats/min. Plain radiographs of the upper extremity only demonstrated soft tissue swelling, and no fracture was detected. He underwent observation and antibiotic treatment at the emergency department. 21 hours later, the symptoms of pain and swelling deteriorated. The temperature of the right forearm skin was significantly lower than that of the left, and his blood pressure started to drop severely. Vascular injuries were suspected based on the clinical symptoms. The patient was transferred to our hospital for further treatment.
After arrival at our hospital, his blood pressure was 120/72mmHg, and his heart rate was 100 beats/min. Physical examination revealed right elbow-joint and forearm pain with swelling, extensive soft tissue injury and numb fingers. The right forearm and hand were cold and pale. The radial pulse was not palpable. Motor function of the right elbow and wrist joint could not be achieved. The patient was taken for emergent angiography to minimize the limb-ischemia time and confirm the injury site.
The patient was transferred to an angiography suite equipped with digital subtraction angiography (Allura Xper FD20, Philips Medical Systems, Best, The Netherlands, or Angiostar Plus, Siemens, Munich, Germany). He was given local anesthesia (10ml of 2% lignocaine) at the artery access site. The right femoral artery was punctured using the Seldinger technique with ultrasound guidance. Selective angiography of the right subclavian artery, which was performed via a 4-Fr vertebral catheter (Cordis, Miami Lakes, FL, USA) through a 6-Fr sheath (Terumo, Tokyo, Japan) from femoral access, showed a complete brachial artery transection at the elbow joint, and the proximal end of the injured artery was blocked by a thrombus. The distal arteries were opacified by collaterals (Figure 1A).
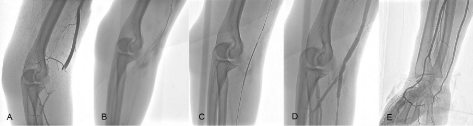
Figure 1: (A) Selective angiography demonstrates complete brachial artery transection at the elbow joint. The proximal end of the injured artery was blocked by a
thrombus. (B) A stiff guidewire is advanced into the proximal end of injured artery and snared with a gooseneck snare through a 4-Fr vertebral catheter. (C) A 6mm
× 80mm bare-metal stent is implanted crossing the brachial artery and ulnar artery. (D,E) After the manual aspiration thrombectomy, angiography shows completely
restored blood flow without contrast extravasation.
To staunch bleeding and minimize the limb-ischemia time, emergent endovascular repair rather than surgical repair was performed. Because passing through the injured site from femoral access was difficult, a transradial pathway was established. Because the right radial pulse was not palpable, it was exposed under local anesthesia. A 0.035-inch, 260-cm long stiff guidewire (Terumo, Tokyo, Japan) was advanced into the proximal end of the injured artery through a 125-cm long 4-Fr vertebral catheter (Cordis, Miami Lakes, FL, USA) from femoral access and was snared with a gooseneck snare (Shanghai Shape Memory Alloy, Shanghai, China) through a 4-Fr vertebral catheter from radial access (Figure 1B). The radial-femoral working wire pathway was established. According to intraoperative angiography, a 6mm × 80mm bare-metal stent (Life Stent, Bard Peripheral Vascular, Tempe, AZ, USA) from radial access over the radial-femoral working wire was implanted to repair the injured brachial artery (Figure1C).
A 6-Fr guiding catheter (Cordis, Miami Lakes, FL, USA) was used to aspirate the artery thrombosis confirmed by arteriography. Manual aspiration was applied via syringe, and the catheter was withdrawn with continuous aspiration. The 6F sheath was then applied to remove the thrombus in the catheter. The following angiography from femoral access demonstrated restored blood flow through the brachial artery and the distal arteries. No contrast extravasation was observed (Figure 1D, Figure 1E). The radial artery access-site incision was sutured after removal of the 6-Fr sheath. Then the femoral access site was wrapped and compressed for 6 hours after removal of the 6-Fr sheath. The procedure lasted 110 minutes. The patient received a total of 4000U of unfractionated heparin by intravenous bolus. Pressure bandages were applied for 24 hours to achieve hemostasis.
Antiplatelet therapy was adopted with clopidogrel (75mg/d) and aspirin (100mg/d). 5000U of low-molecular-weight heparin were administered twice a day. Physical examination revealed a palpable radial pulse. The temperatures of the right forearm and hand skin were similar to those of the left forearm and hand skin. The numbness and swelling were significantly relieved 4 days after the operation. Motor function of right-upper extremity was slightly limited. No treatment-related complications were noted during hospitalization. The patient was discharged 25 days after admission. The patient received clopidogrel (75mg/d) and aspirin (100mg/d) for 1 month after discharge. The motor function of the right-upper extremity was recovered at the 3-month follow-up. During the 5-year followup period, the stent fracture was not observed, and angiography confirmed nonhemodynamic stenosis caused by neointimal hyperplasia (Figure 2A-2F). Considering the satisfactory blood flow and few overt symptoms, no further intervention was performed.
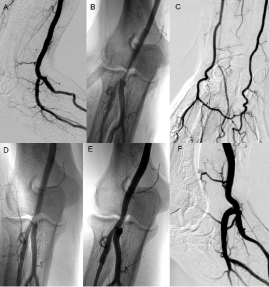
Figure 1: (A) Angiography shows satisfactory blood flow without contrast
extravasation at the 16-day follow-up. (B,C) Angiography indicates in-stent
stenosis of 50% at the 8-month follow-up. The bare-metal stent is embedded
in the neointimal hyperplasia. There is satisfactory blood flow at the distal
arteries. No obvious abnormal symptom was found. (D) Angiography after 18
months demonstrates satisfactory blood flow without contrast extravasation.
Vascular remodeling and alleviated stenosis is observed. (E,F) 5 years later,
the thickened artery wall does not change significantly. Angiography shows
satisfactory blood flow in the brachial artery without contrast extravasation.
Importantly, no sign of stent fracture appears.
Discussion
Manifestations of blunt vascular injury are often clinically occult, making initial diagnosis difficult [3]. The brachial artery injury and complicated artery embolization may not be appreciated initially, leading to a 21-hour delay in diagnosis and treatment. The hemodynamical instability and severe soft-tissue injury strongly suggest a staged approach with emergent endovascular therapy to avoid a delay related to anesthesia and transport. Because the injury site was located at the elbow, a flexible bare-metal stent was implanted to avoid the occlusion of the main vessel branch and stent fracture. Then the thrombus was removed by catheter thrombectomy. As satisfactory blood flow was obtained and no contrast extravasation was detected by angiography, we decided to perform second-stage open surgery only if reexamination would show undesirable results. A 5-year follow-up proves that this “one-stop” endovascular treatment strategy is feasible and effective in unstable patients with brachial artery injuries and thrombus at the elbow.
Brachial artery injuries account for 15%-30% of all peripheral arterial injuries [4]. Prompt treatment is required to reduce the likelihood of amputation[1]. Generally, surgical repair is suggested to repair vessel transection. Because this patient presented with severe soft-tissue injury, open repair was a challenging operation, especially in the presence of hemorrhagic shock. However, it could be dangerous to approach and challenging to access the arterial trauma in open surgery due to a distorted local anatomy. It may prolong ischemic complications and increase the risk of iatrogenic nerve injury, particularly when compromised by the presence of an associated hematoma and soft-tissue injury [5]. Moreover, open operation around a joint with blunt injury with may lead to synarthrophysis. Due to the thrombus, additional endovascular techniques might be required to treat distal embolic complications of the primary injury.
The “one-stop” endovascular treatment strategy combines endovascular stent implantation and thrombectomy, which can maintain the vascular integrity and remove the thrombus with a shorter operative time, decreased operative blood loss and lower complication rates [2, 5, 6]. We finally decided to perform emergent one-stage endovascular treatment to restore the blood flow. When endovascular repair fails, traditional surgical repair could be performed. If severe complications were found in the follow-up, a second open-surgery procedure may be suggested.
Endovascular repair of complete artery transection remains challenging. Some authors suggest that dual femoral and brachial access “rendezvous” techniques may be necessary for adequate guidewire manipulation and endo-graft positioning [7]. In this case, the “rendezvous” technique was adopted to recanalize complete brachial artery transection. A radial-femoral wire pathway was established with a guidewire, which was introduced through the femoral access and snared with a gooseneck snare from radial access. Because the lesion site was close to the bifurcation of the ulnar and radial arteries, no adequate vascular fixation site existed at the distal brachial artery. We decided to implant the stent crossing the brachial artery and ulnar artery. Therefore, the stent’s impact on the ulnar artery blood flow must be considered. With a coated stent, the potential to cover the origin of the ulnar artery, leading to acute ischemia, remains [8]. A bare-metal stent was finally chosen to repair the brachial artery.
Unlike a coated-stent graft, the bare-metal stent acts as a passive barrier by reducing blood flow near the injury site [9]. Moreover, the hemodynamic effects of a bare-metal stent may promote the activation and aggregation of platelets [10]. Subsequently, the thrombus formation will eventually stop the bleeding. Based on intraoperative arteriography, the stent’s diameter was oversized by 10% to ensure a seal, and the length was required to cover the injured site [2].
After the stent implantation, thrombosis in the injured artery was treated with manual aspiration thrombectomy, which is a well After the stent implantation, thrombosis in the injured artery was treated with manual aspiration thrombectomy, which is a wellaccepted approach for the treatment of an acute large-vessel thrombus [11]. It allowed definitive treatment, leading to a desire to attempt to treat it in one session.
Arteriography was subsequently performed to determine whether another stent was necessary after the first stent placement, which showed restored blood flow in injured brachial, ulnar, radial and distal arteries. Importantly, no contrast extravasation was found. The hemodynamical disorder and clinical symptoms improved gradually. There was no contrast extravasation and stent fracture in the following reexaminations. Angiography showed completely restored blood flow without stenosis at the 16-day follow-up. A nonhemodynamic stenosis of 50% was found at the 8-month follow-up. The acute expanding force of the self-expanding stent may be the key cause of neointimal hyperplasia [12]. Previous studies have indicated a comparatively high rate of late clinical failure after stent implantation in treatment of chronic limb ischemia [12]. In contrast, the stenosis of this case was alleviated at the 18-month and 5-year follow-ups. Unlike patients with atherosclerotic stenosis, the injured artery’s thickness is generally in the physiological range. Rather than dilating the artery lumen, the stent was applied mainly to cover the injury site, which did not impose high stress on the artery wall. As stent forces alone are an important risk for vessel-wall response, low-strain is related to relatively low levels of hyperplastic or hypertophic activity. Therefore, intimal thickening did not obviously increase [13]. Additionally, as the crucial mechanical-chemical signal transduction transmission remains [14]. the vascular endothelium might be directly stimulated by joint motion. Moreover, the special local hemodynamics near the joint may alter the blood-flow patterns and wall shear stress frequently, possibly leading to vascular adaptation and remodeling [14].
Although the stent may be prone to fracture because of mechanical forces by the motion of the elbow joint, the endovascular repair seems to be a promising strategy to stop bleeding without any structural damage. We did not observe any stent fracture during the 5-year follow-up period. Several protective factors may exist. First, with the improvement of manufacturing processes and updated materials, the stents have demonstrated improved radial strength and the ability to recover from being crushed [15]. Second, the wall of the injured branchial artery was in the normal range. The stress effects of artery on the implanted stent was low. Third, the implanted stent was shorter than 10cm and was more resistant to fracture. Fourth, the neointimal hyperplasia around the bare-metal stent may be a critical protective factor to maintain its integrity. These properties may lead to less risk of fracture although the underlying causes remain undetermined.
Conclusion
This case report may lead to the following conclusions: 1. A “one-stop” endovascular treatment strategy incorporating stent implantation and embolectomy can provide a suitable alternative to surgical repair to treat a severed vascular injury complicated by a thrombus; 2. A bare-metal stent may be a suitable alternative for endovascular repair for artery transection at the joint, particularly with a high risk of coverage of branch vessels. Further multicenter prospective research for these patients is required to define the role of this treatment modality in a trauma setting.
Conflict of Interest or Funding Statement
This study has received funding by grants from Key Projects of PLA(CJN14zJ010).
References
- Wolma FJ, Larrieu AJ, Alsop GC. Arterial injuries of the legs associated with fractures and dislocations. Am J Surg. 1980; 140: 806-809.
- Branco BC, Boutrous ML, DuBose JJ, Leake SS, Charlton-Ouw K, Rhee P, et al. Outcome comparison between open and endovascular management of axillosubclavian arterial injuries. J Vasc Surg. 2016; 63: 702-709.
- Mills WJ, Barei DP, McNair P. The value of the ankle-brachial index for diagnosing arterial injury after knee dislocation: a prospective study. J Trauma. 2004; 56: 1261-1265.
- Vu T, Asensio JA, Mazzini FN, Sciarretta JD, Chandler J, Lieberman EH, et al. Brachial vessel injuries: high morbidity and low mortality injuries. Eur J Trauma Emerg Surg. 2011; 37: 459.
- Xiao L, Shen J, Tong JJ. Emergency stent-graft implantation for iatrogenic peripheral arterial rupture. Radiol Med. 2013; 118: 152-157.
- Branco BC, DuBose JJ, Zhan LX, Hughes JD, Goshima KR, Rhee P, et al. Trends and outcomes of endovascular therapy in the management of civilian vascular injuries. J Vasc Surg. 2014; 60: 1297-1307.
- Chemelli AP, Wiedermann F, Klocker J, Falkensammer J, Strasak A, Czermak BV, et al. Endovascular management of inadvertent subclavian artery catheterization during subclavian vein cannulation. J Vasc Interv Radiol. 2010; 21: 470-476.
- Stahnke M, Duddy MJ. Endovascular repair of a traumatic axillary pseudoaneurysm following anterior shoulder dislocation. Cardiovasc Intervent Radiol. 2006; 29: 298-301.
- Sultan S, Hynes N. Multilayer flow modulator stent technology: a treatment revolution for US patients? Expert Rev Med Devices. 2015; 12: 217-221.
- Biasetti J, Gasser TC, Auer M, Hedin U, Labruto F. Hemodynamics of the normal aorta compared to fusiform and saccular abdominal aortic aneurysms with emphasis on a potential thrombus formation mechanism. Ann Biomed Eng. 2010; 38: 380-390.
- Lapergue B, Blanc R, Gory B, Labreuche J, Duhamel A, Marnat G, et al. Effect of Endovascular Contact Aspiration vs Stent Retriever on Revascularization in Patients With Acute Ischemic Stroke and Large Vessel Occlusion: The ASTER Randomized Clinical Trial. Jama. 2017; 318: 443-452.
- Cha SH, Han MH, Choi YH, Yoon CJ, Baik SK, Kim SJ, et al. Vascular responses in normal canine carotid arteries: comparison between various self-expanding stents of the same unconstrained size. Invest Radiol. 2003; 38: 95-101.
- Freeman JW, Snowhill PB, Nosher JL. A link between stent radial forces and vascular wall remodeling: the discovery of an optimal stent radial force for minimal vessel restenosis. Connect Tissue Res. 2010; 51: 314-326.
- Ando J, Yamamoto K. Effects of shear stress and stretch on endothelial function. Antioxid Redox Signal. 2011; 15: 1389-1403.
- Schillinger M, Sabeti S, Loewe C, Dick P, Amighi J, Mlekusch W, et al. Balloon angioplasty versus implantation of nitinol stents in the superficial femoral artery. N Engl J Med. 2006; 354: 1879-1888.