
Research Article
Austin J Clin Med. 2024; 9(1): 1045.
Utility of Platelet Indices in Critically ill Children
Nagwan Y Saleh*; Ahmed A Khatab; Muhammad S El-Mekkawy; Shaimaa M Nomair
Department of Pediatrics, Faculty of Medicine, Menoufia University, Shebin Elkom, Egypt
*Corresponding author: Nagwan Yossery Saleh Corresponding author. Professor of Pediatrics, Faculty of Medicine, Menoufia University Hospital, Yassin Abdel-Ghafar Street, Shebin El-kom, Menoufia, 32511, Egypt. Tel: + 201060408035 Email: drnagwan80@gmail.com
Received: March 04, 2024 Accepted: April 09, 2024 Published: April 16, 2024
Abstract
Background: Platelet indices have been recently used to predict outcomes for critically ill patients but Pediatric data is limited. We aimed to evaluate role of platelet indices in diagnosis of sepsis and predicting prognosis among critically ill children.
Methods: This was prospective observational study conducted on 133 children admitted to Pediatric Intensive Care Unit (PICU) of a tertiary center. Patients were evaluated on admission by routine laboratory biomarkers and clinical risk score, in addition to platelet indices, namely Mean Platelet Volume (MPV), Platelet Distribution Width (PDW), and Plateletcrit (PCT). Patients were followed up till hospital discharge. Primary outcome was PICU mortality.
Results: 133 patients were recruited.47.4% had sepsis; 7.5% had non-infectious Systemic Inflammatory Response Syndrome (SIRS); and 45.1% had no SIRS. No Significant difference in all platelet indices was observed between sepsis, non- infectious SIRS, and non-SIRS. MPV was significantly higher among non-survivors compared with survivors [median and IQR: 8.2(7.7–9.9)] but no significant difference was found between the two groups in PCT and PDW. Serum albumin, platelet count, and WBC were significantly lower among non-survivors.Multivariate logistic regression analysis revealed that mechanical ventilation and serum albumin are independent predictors of mortality [ORand 95% CI: 37.1(4.4–311.7) and 0.26(0.09–0.72) respectively]. MPV and PDW were positively correlated, while PCT were negatively correlated, with pSOFA score [Rs: 0.21, 0.17, -0.22; p=0.017, 0.048, 0.009].
Conclusion: Platelet indices have no value for diagnosis of sepsis but they possess some prognostic value. However, mechanical ventilation and serum albumin are independent far superior as prognostic indicators.
Keywords: Mean platelet volume; Mortality; Pediatric; Plateletcrit; Platelet distribution width
Introduction
Critical illness is made up of a heterogeneous group of disorders that share a risk of organ dysfunction, long-term morbidity, and mortality [1]. Approximately 80% of the patients admitted into Intensive Care Units (ICU) survive the acute event, and most remain in this unit briefly. However, a subgroup does not recover sufficiently quickly to become independent and from then they recover slowly, these patients are called Chronically Critically Ill (CCI) patients who comprise 5 to 10% of the patients admitted into Intensive Care Units [2].
The outcome of critically ill children recovering from life threatening diseases in intensive care situations has improved owing to advancing diagnostic and therapeutic methods. Clinicians recognized the importance of identifying patients with the highest risk of mortality among those admitted to the PICU, and of proper monitoring and appropriate intervention and treatment [3].
Pediatric critical illness can profoundly disrupt child health and development and negatively affect family function and well-being. Although (PICU) mortality is declining, a growing number of survivors develop deficits that persist beyond hospital discharge [4].
Platelet (PLT), a major and essential constituent of blood, plays an important role in physiological and pathological processes such as coagulation, thrombosis, inflammation and maintenance the integrity of vascular endothelial cells, by mediate leukocyte movement from the bloodstream through the vessel wall to tissue; platelets also secrete microbicidal proteins and antibacterial peptides [5].
Platelet indices are biomarkers of platelet activation. They allow extensive clinical investigations focusing on the diagnostic and prognostic values in a variety of settings without bringing extra costs. Among these platelet indices Plateletcrit (PCT), Mean Platelet Volume (MPV) and Platelet Distribution Width (PDW) are a group of platelet parameters determined together in automatic Complete Blood Count (CBC) profiles, they are related to platelet’s morphology and proliferation kinetics [6].
When platelet production is decreased, young platelets become bigger and more active, and MPV levels increase. Increased MPV indicates increased platelet diameter, which can be used as a marker of production rate and platelet activation [7].
PDW is numerically equal to the coefficient of PLT volume variation, which is used to describe the dispersion of PLTs volume [8]. Platelets play an important role in inflammation, and recently, several additional functions for platelets in the process of inflammation were defined [9]. The objective of the present study was to assess the role of platelet indices in diagnosis and prognosis of critically ill children in Pediatric Intensive Care Unit
Subjects and Methods
The Design of the Study
In this prospective study, we enrolled 133 critically ill children admitted to a 10-bed PICU at Menoufia University Hospital, Egypt, from March 2019 to September 2020. The Scientific and Ethical Committee approved the study protocol of Menoufia University, and informed consent was obtained from parents before enrolling their children in the study. Critically ill children in the PICU aged 1 month to 18 years were included in this study. Exclusion criteria were 1) age less than one month or more than 18 years and 2) Any children with aplastic anemia or received immunosuppression drugs or platelets disorders and oncology patients with bone marrow depression and 3) inability to follow up for the first 30 days after discharge.
The PICU Patients
The included patients were diagnosed according to the International Pediatric Sepsis Consensus Conference, characterizing, sepsis, non-infectious SIRS, and non-SIRS [10]. Sepsis is a systemic response to an infectious stimulus characterized by two or more of the following, resulting in infection: (a) a temperature of more than 38°C or less than 36°C, (b) pulse rate > 90 beats/minute, (c) breath rate > 20 breaths /minute or PaCO2 < 32 mm Hg, and (d) White Blood Cell count (WBC) of > 12000/mm3 or < 4000/mm3, or > 10% immature (band) formation in a total blood count.
The Outcomes of the Study
The primary outcome measure was PICU mortality during hospital admission or during the 30-days follow up period after hospital discharge. The length of stay (PICU and hospital), need and duration of Mechanical Ventilation (MV) were secondary outcomes.
The Method of the Study
We collected the complete history of all children including in this study, including age, sex, admission data, and length of stay in the PICU and the inpatient department. Vital signs, anthropometric measurements, and examination of all body systems were also assessed. PICU scoring systems were applied, including the 1) Pediatric Risk of Mortality score (PRISM) 2) Pediatric Index of Mortality-2 (PIM2), and 3) Pediatric Sequential Organ Failure Assessment Scale (pSOFA). The PRISM score was calculated within 24 hours of admission for each patient, using 14 clinical and laboratory variables. Values for these variables were entered into the PRISM application (http://www.sfar.org/scores2/prism2.php), which calculates the expected death rate [11]. PIM2 is a more rapid technique for which scores are estimated within 1 hour of in-person contact with the patient, and scores correspond to a predicted mortality rate [12]. The pediatric Sequential Organ Failure Assessment Scale (pSOFA) is used to assess organ dysfunction. Depending on the patient's baseline risk level, a pSOFA score of 2 or greater corresponds to a 2- to 25-fold greater risk of death than patients with pSOFA scores was less than 2 [13].
Arterial blood gases, random blood glucose, and Complete Blood Count (CBC) were analyzed (Pentra ABX 80 analyzer; Horiba, Paris, France). C-Reactive Protein (CRP), hepatic function (alanine aminotransferase and aspartate aminotransferase) was determined using a kinetic UV-optimized IFCC method (LTEC Kit, England). Renal function (blood urea and serum creatinine) was determined colorimetrically (Diamond Diagnostic kit, Germany). Blood culture, chest X- radiography, brain CT, and other laboratory or radiological analyses were performed as needed.
The procedure: Platelet Distribution Width (PDW): is a regular parameter in blood routine examination which reflects variation of platelet size distribution with a range from 8.3% to 56.6%. Mean Platelet Volume (MPV): MPV was calculated by the following formula: MPV (fL) = [(PCT (%)/platelet count (×10 /l)] × 105. Plateletcrit (PCT): is the volume occupied by platelets in the blood as a percentage and calculated according to the formula PCT = platelet count × MPV / 10,000 (within 24 hours of PICU admission).
Statistical Analysis
Data were statistically analyzed using SPSS (version 19, SPSS Inc, Chicago, Illinois). Descriptive statistics included arithmetic medians and Interquartile Ranges (IQRs) of quantitative data and numbers and percentages of qualitative data. Analytical statistics included the Chi-square (Χ2) test, Student's t-test, Mann-Whitney test, and Fisher's exact test. We used logistic regression models to determine the ability of platelets indices to predict mortality. Receiver operating characteristics (ROC) analysis was performed for the diagnostic and prognostic powers of the platelet’s indices, and other variables. P-values < 0.05 were considered significant.
Results
Table 1 showed demographic and clinical data of survivors and non-survivors. Non-survivors had significantly higher frequency of severe sepsis, Acute Respiratory Distress Syndrome (ARDS), mechanical ventilation, Multiple Organ Dysfunction Syndrome (MODS) and nosocomial infections. Non survivors also had significantly higher pSOFA score, PRISM, and longer duration of mechanical ventilation.
Variable
Survivors (n=105)
Non-survivors (n=28)
P
valueAge, month
24 (8 – 72)
18 (7.3 – 117)
0.88
Male sex
52 (49.5%)
11 (39.3%)
0.34
Weight, Kg
12 (7 – 18)
9.3 (6.6 – 22)
0.71
Height, cm
85 (63.5 – 109.5)
79 (63.5 – 116.8)
0.92
BMI
16.3 (14.8 – 18.5)
16.2 (14.1 – 18.9)
0.59
Malnutrition
48 (45.7%)
18 (64.3%)
0.08
Category
- Sepsis
- Non-infectious SIRS
- Non-SIRS
47 (44.8%)
6 (5.7%)
52 (49.5%)
16 (57.1%)
4 (14.3%)
8 (28.6%)
0.08
Shock on admission
29 (27.6%)
12 (42.9%)
0.12
Severe sepsis
7 (6.7%)
12 (42.9%)
<0.001*
MODS
28 (26.7%)
21 (75%)
<0.001*
ARDS
1 (0.9%)
10 (35.7%)
<0.001*
MV
23 (21.9%)
25 (89.3%)
<0.001*
MV duration
0 (0 – 4.5)
4 (0 – 16)
<0.001*
Nosocomial infection
17 (16.2%)
15 (53.6%)
<0.001*
PRISM mortality risk%
2 (1.4 – 4.2)
3.7 (1.8 – 10.4)
0.012*
PIM2 mortality risk%
2.3 (1.7 – 6.2)
5.4 (1.8 – 14.6)
0.057
pSOFA
5 (4 – 6)
6 (5 – 9.8)
0.002*
BMI: Body Mass Index; SIRS: Systemic Inflammatory Response Syndrome; PICU: Pediatric Intensive Care Unit; PRISM: Pediatric Risk of Mortality; PIM2: Pediatric Index of Mortality2; pSOFA: Pediatric Sequential Organ Failure Assessment Score; ARDS: Acute Respiratory Distress Syndrome; MODS: Multiple Organ Dysfunction Syndrome; MV: Mechanical Ventilation *Statistically Significant.
Table 1: Demographic and clinical data of survivors and non-survivors.
Laboratory data of survivors and non-survivors is shown in Table 2. Non-survivors had significantly higher CRP and MPV level while WBC, Platelet count and albumin were statistically significant lower in non- survivor group. No statistically significant differences were found between 2 groups regarding other platelet indices or other laboratory variables.
Variable
Survivors
(n=105)Non-survivors
(n=28)P
valueCRP, mg/dL
16 (5 – 48)
32.5 (12.3 – 87.5)
0.013*
Hemoglobin, g/dL
10.7 (9.4 – 12.2)
10.3 (8.9 – 10.9)
0.10
WBC, 1000/uL
13.1 (9 – 17.7)
9.9 (5.9 – 15.9)
0.044*
Platelets, 1000/uL
298 (181 – 383.5)
202.5 (130.5 – 330.3)
0.027*
Creatinine, mg/dL
0.4 (0.3 – 0.6)
0.5 (0.3 – 0.7)
0.25
ALT, U/L
24 (15 – 51)
37 (19 – 77)
0.063
Albumin, g/dL
3.8 (3.3 – 4.2)
2.7 (2.2 – 3.1)
<0.001*
Bilirubin, mg/dL
0.4 (0.2 – 0.7)
0.4 (0.23 – 1.35)
0.56
Base excess
-5.3 (-8.6 – -2.2)
-4.7 (-11.1 – 0.15)
0.68
ANC, 1000/ml
7.6 (4.8 – 12.1)
5.3 (2.9 – 9.4)
0.14
MPV
7.7 (7.2 – 8.8)
8.2 (7.7 – 9.9)
0.015*
PDW
10.8 (9.9 – 11.9)
11.3 (10.6 – 13.2)
0.067
PCT
0.21 (0.14 – 0.31)
0.19 (0.12 – 0.24)
0.15
CRP: C-Reactive Protein; WBC: White Blood Cell Count; ANC: Absolute Neutrophilic Count; ALT: Alaninine Aminotransferase; MPV: Mean Platelet Volume; PDW: Platelet Distribution Width; and PCT: Plateletcrit; *Statistically Significant.
Table 2: Laboratory data of survivors and non-survivors.
Correlation of PDW, MPV and PCT with other variables. PDW was positively correlated with pSOFA, MPV, PCT, Neutrophil count, WBC, platelet count, creatinine, ALT and serum albumin. MPV was positively correlated with pSOFA, PDW, PCT, Neutrophil count, WBC, platelet count, creatinine, ALT and serum albumin. PCT was positively correlated with pSOFA, MPV, PDW, lymphocytes, WBC, platelet count, creatinine, ALT and serum albumin (Table 3).
Variable
PDW
MPV
PCT
Rs
P-value
Rs
P-value
Rs
P- value
Age
0.092
0.29
0.03
0.76
-0.15
0.09
Weight
0.093
0.28
0.02
0.87
-0.15
0.086
PRISM
0.003
0.97
0.04
0.67
-0.11
0.21
PIM2
0.028
0.76
0.06
0.52
-0.03
0.75
pSOFA
0.17
0.048*
0.21
0.017*
-0.22
0.009*
PICU stay
-0.04
0.66
0.07
0.41
0.08
0.37
MV duration
0.02
0.81
0.11
0.19
0.08
0.38
Vasoactive infusion days
0.02
0.79
0.15
0.89
0.001
0.99
MPV
0.81
<0.001*
NA
NA
-0.28
0.001*
PDW
NA
NA
0.81
<0.001*
-0.32
<0.001*
PCT
-0.32
<0.001*
-0.28
0.001*
NA
NA
Neutrophil
-0.18
0.034*
-0.18
0.039*
0.13
0.14
Lymphocytes
-0.066
0.45
-0.01
0.89
0.18
0.037*
WBC
-0.25
0.003*
-0.23
0.009*
0.22
0.011*
Platelet
-0.52
<0.001*
0.50
<0.001*
0.91
<0.001*
Hemoglobin
-0.04
0.62
-0.05
0.57
0.08
0.37
Base excess
0.035
0.68
0.028
0.75
0.09
0.29
CRP
0.099
0.26
0.07
0.42
-0.07
0.45
Creatinine
0.23
0.007*
0.24
0.007*
-0.23
0.007*
ALT
0.23
0.007*
0.25
0.005*
0.03
0.75
Albumin
-0.28
0.004*
-0.24
0.015*
0.28
0.005*
Total bilirubin
0.077
0.38
0.06
0.53
0.03
0.78
*Statistically Significant PICU: Pediatric Intensive Care Unit; PRISM: Pediatric Risk of Mortality; PIM2: Pediatric Index of Mortality2; pSOFA: Pediatric Sequential Organ Failure Assessment Score; MV: Mechanical Ventilation; CRP: C-Reactive Protein; WBC: White Blood Cell Count; ANC: Absolute Neutrophilic Count; ALT: Alaninine Aminotransferase; MPV: Mean Platelet volume; PDW: Platelet Distribution Width; PCT: Plateletcrit
Table 3: Correlation of PDW, MPV and PCT with other variables.
Univariate and multivariate logistic regression analyses for the prediction of mortality by different variables are shown in Table 4. Univariate logistic regression analysis for prediction of mortality showed that PRISM, pSOFA, MV, ARDS and severe sepsis were positively associated with mortality. Also, CRP, Platelet count and serum albumin were positively associated with mortality. Multivariate logistic regression analysis showed that mechanical ventilation and serum albumin were independent predictors of mortality.
Univariate logistic regression analysis
Variable
Odds ratio (95% CI)
P-value
PRISM
1.07 (1.01 – 1.13)
0.015*
pSOFA
1.30 (1.12 – 1.5)
0.001*
MV
29.7 (8.2 – 107.3)
<0.001*
ARDS
57.8 (6.9 – 479.3)
<0.001*
Severe sepsis
10.5 (3.6 – 30.7)
<0.001*
CRP
1.01 (1.002 – 1.019)
0.019*
WBC
0.99 (0.95 – 1.037)
0.67
Platelet count
0.996 (0.99 – 1.0)
0.028*
Albumin
0.24 (0.12 – 0.48)
<0.001*
MPV
0.99 (0.94 – 1.07)
0.96
Multivariate logistic regression analysis
Variable
Adjusted odds ratio (95% CI)
P value
PRISM
0.98 (0.86 – 1.12)
0.84
pSOFA
0.92 (0.69 – 1.23)
0.59
MV
37.1 (4.4 – 311.7)
0.001*
Albumin
0.26 (0.09 – 0.72)
0.01*
CRP
1.006 (0.99 – 1.02)
0.43
Platelets
0.996 (0.99 – 1.002)
0.25
ARDS
847241927 (0.0 - .)
0.99
Severe sepsis
2.93 (0.41 – 20.8)
0.28
PRISM: Pediatric Risk of Mortality; pSOFA: Pediatric Sequential Organ Failure Assessment Score; MV: Mechanical Ventilation; ARDS: Acute Respiratory Distress Syndrome; CRP: C-Reactive Protein; WBC: White Blood Cell Count; MPV: Mean Platelet Volume; OR (95% CI): Odds Ratio and 95% Confidence Interval; *Statistically Significant.
Table 4: Univariate and Multivariate logistic regression analysis for prediction of mortality by different clinical variables.
ROC curve analysis showed CRP and MPV had the largest area under the curve for prediction of mortality followed by platelets, then serum albumin. CRP level had a sensitivity of 71.4% and a specificity of 54.3% for prediction of mortality. MPV level had a sensitivity of 67.9% and a specificity of 59%for prediction of mortality (Table 5 and Figure 1A and B).
Variable
AUC (95% CI)
P
valueCutoff level
Sensitivity
Specificity
MPV
0.65 (0.54 – 0.76)
0.015*
= 7.95
67.9%%
59%
PDW
0.61 (0.50 – 0.72)
0.067
= 10.5
78.6%
41%
PCT
0.59 (0.48 – 0.70)
0.15
= 0.17
46.4%
71.4%
CRP, mg/dL
0.65 (0.54 – 0.77)
0.012*
= 21.5
71.4%
54.3%
WBC,1000/uL
0.37 (0.24 – 50)
0.044*
= 38.6
7.1%
99%
Platelets,1000/uL
0.64 (0.53 – 0.75)
0.027*
= 213.5
57.1%
66.7%
Albumin, g/dL
0.62 (0.52 – 0.72)
0.052
= 3.2
82.1%
55.8%
CRP: C-Reactive Protein; WBC: White Blood Cell Count; MPV: Mean Platelet Volume; PDW: Platelet Distribution Width; PCT: Plateletcrit; *Statistically Significant.
Table 5: ROC curve analysis for prediction of mortality by different laboratory variables.
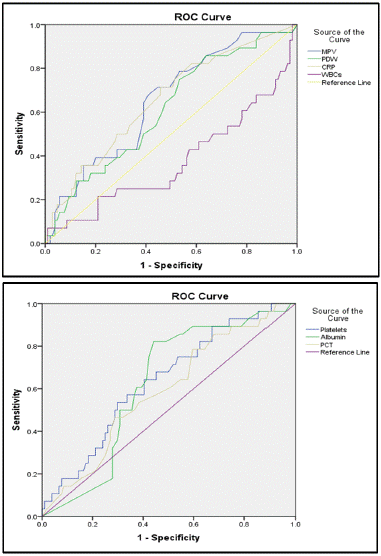
Figure 1: A) ROC curve analysis for prediction of mortality by MPV, PDW, CRP, and WBCs. B) ROC curve analysis for prediction of mortality by platelets, albumin, and PCT.
Discussion
Platelet indices are a group of parameters that are used to measure the total amount of PLTs, PLTs morphology and proliferation kinetics [14]. The commonly used PLT indices include PLT count, Mean Platelet Volume (MPV), Platelet Distribution Width (PDW), and Platelet-Crit (PCT) [15].
The nature of MPV as an inflammatory marker has been suggested by previous study [16]. In recent years, the number of studies suggesting that the platelet and their indices can be used as inflammatory markers in cancer cases in addition to cardiovascular, cerebrovascular, inflammatory and thromboembolic diseases has been increasing by the time [9].
Our study showed that platelet indices are not sensitive markers for diagnosis of sepsis among critically ill children. No significant differences were found between patients with sepsis, non-infectious SIRS, and non-SIRS regarding platelet indices.
In contrast to our results, Leile and Borne, [17] showed a higher MPV in patients with sepsis than in patients with localized infection and suggested that an increase of MPV in patients with bacterial infection could indicate the occurrence of septicemia. Also, Guclu et al., [14] demonstrated that MPV and PDW were significantly higher among septic patients. In addition, a lower platelet count was observed in septic patients. Another study in children conducted by Fioretto et al., [18] reported PCT was better than CRP for diagnosing sepsis and septic shock, mainly at admission, and is related to disease severity.
The discrepancy between our study and the other studies could be explained by the small sample size or heterogeneity in the reasons for PICU admission.
In our study, CRP was significantly higher among patients with sepsis, which makes it more useful for sepsis diagnosis. Surprisingly, CRP was higher among patients with non-infectious SIRS. CRP is an inflammatory marker and can rise in SIRS even without infection. We diagnosed sepsis according to SIRS criteria combined with proven or suspected infection [10], and it is possible that our current tools are not sensitive enough for diagnosis of hidden infection, particularly in light of the severe lack of resources.
In comparison, several other studies [19-21] demonstrated that CRP is equally or more useful than PCT for diagnosing sepsis and septic shock. Also, Farag et al., [22] detected in this study that the serum CRP levels on admission, 2nd day, and 4th day following admission were significantly higher in sepsis compared to non-infectious SIRS.
The overall mortality rate for children in our study was 20.3 %, which was close to a mortality rate of 27.8% reported by Sriram et al., [23], whereas it ranged between 16.8 to 31.8% in a study by Martin et al., [24].
The explanation of wide variability in mortality rates in different PICUs worldwide can be attributed to differences in population characteristics in terms of admission pathologies in PICUs, co-morbidities and health systems (fragmentation, accessibility, available resources and social sanitary conditions).
The association between platelet indices and mortality in septic shock patients is unclear. For example, increased MPV has been associated with mortality in adult septic shock patients [25].
Our study showed that platelet count on PICU admission was significantly associated with mortality, but that platelet indices such as MPV and PDW were not associated with mortality in these patients. This study found that, platelet count was significantly lower among non-survivor group. This consistent with Choi et al., [26] who found platelet count was 3-fold higher in survivors than in non-survivors. Similarly, Dellinger et al., [27] showed that low platelet count was associated with more severe illness and had higher risk of mortality.
Also, El-Mashad et al., [28] revealed that, platelet count was significantly increased among survivors than non-survivors’ group. Likewise, Vanderschueren et al., [29] showed that in adults admitted in the ICU; patients who died had a lower platelet count than survivors.
The low platelet count in non-survivors may be attributed to the depletion of coagulation factors and platelet consumption during the septic process
In our study, non-survivors had statistically significant higher MPV level compared with survivors, suggesting that MPV can be used as a novel prognostic indicator in critically ill patients. In the same line Becchi et al., [30] found that the average MPV gradually increased in non-survivors, whereas it decreased in survivors. On the contrary, Choi et al., [26] found MPV was higher in survivors, but the differences were not statistically significant. Also, Golwala et al., [8] found no difference in the MPV between the dead and the survivors.
In our study; MPV was not predictor of mortality. This shows MPV may not be useful as a prognostic marker of mortality. In contrast, Purbiya et al., [5] found that higher MPV was associated with increased risk of death. Another retrospective analysis of Zhang et al., [15] showed that high MPV value and high PDW value were associated with more severe illness and had higher risk of mortality. Golwala et al., [8] found that platelet indices and their ratios were useful predictors of mortality. In addition, Purbiya et al., [5] and Zhang et al., [15] studies confirm that platelet indices are useful to predict mortality.
Explanation of this discrepancy could be attributed to differences in illness severity between their population and ours.
Our study showed that, no statistically significant difference was found between survivors and non survivors regarding PDW and PCT. Also, Choi et al., [26] found PDW was higher in survivors, but the differences were not statistically significant. Also, Patrick and Lazarchick, [31] did not find a difference between the PDW of those who died compared with survivors. They studied PDW in neonates with late onset sepsis. They found that PDW increased with sepsis. In contrast, Purbiya et al., [5] demonstrated that, statistically significant difference was found between the groups regarding PDW and PCT. Also, Golwala et al., [8] found PCT in those who died was significantly different from those who survived. Low PCT in the non-survivors may be attributed to the fact that, PCT is influenced by number and size of platelets.
Consistent with our findings, Abd-Elmoneim et al., [16] demonstrated that MPV or platelet count alone could not predict shock and 28-day mortality in patients with severe sepsis.
In our study, non–survivors had statistically significant higher PRISM mortality risk. This agreed with Choi et al., [26] who found that, PRISM score was significantly greater in the non-survivors than in survivors. Also, with El-Mashad et al., [28] who showed that PRISM mortality risk statistically significantly higher among non-survivors than survivors’ group.
In the present study, we found that serum albumin level was significantly lower among non-survivor group while Ye et al., [32] found no significant difference between survivors and non survivors regarding albumin.
The current findings also, in the same line with, Girish and Soumya, [33] found low serum albumin level to be a significant predictor of mortality. Because albumin is a marker for nutritional status, inflammatory response, and the severity of the disease, low serum albumin values are believed to be a significant factor for increased morbidity and mortality in children followed up in the PICU.
Many studies have demonstrated that mechanical ventilation is associated with complication; the weaning period may comprise up to 40% of MV days [34]. Epstein et al., [35] reported that weaning from MV is difficult for 30% of patients, and such patients showed a higher mortality rate and that study agreed with our results regarding mechanical ventilation as independent predictor of mortality in PICUs.
This study showed that, MPV was positively correlated with PCT, platelet count, and serum albumin. In the same line, Abd-Elmoneim et al., [16] showed that, the rise in MPV is related to the increase in PCT, which is a strong indicator of sepsis. Also, Leile and Borne, [17] found MPV and PDW are directly related. When there is an increase in PDW, MPV increases.
In contrast to our findings, Kim et al., [36] found MPV has no significant correlation to platelet count. This may be explained by the difference in pathophysiology of sepsis between adult and pediatric populations where platelets respond to sepsis through increase in size with no significant changes in count.
In the current study, PDW was positively correlated with MPV. In the same line Abd-Elmoneim et al., [16] reported that, there were positive correlations between MPV and PDW 24, 48, and 72 h after emergency department admission.
We also found that PCT was positively correlated with platelet count. This agreed with Gao et al., [37] found that PCT was positively correlated to platelet count.
Univariate logistic regression analysis showed that mechanical ventilation, CRP, Platelet count and serum albumin were predictors of mortality. However, multivariate analysis showed that only mechanical ventilation and albumin level were the only independent predictors of mortality.
Our study demonstrated that MPV and CRP achieved the highest AUC for prediction of mortality, followed by platelet count. The AUC related to PCT and PDW was not significant.
Another study by Sayed et al., [9] reported that CRP had optimal cutoff = 96 with sensitivity of 57.89% and a specificity of 85.37%. Choi et al., [26] demonstrated higher sensitivity and a specificity than ours regarding platelet count 85.7%. While, Purbiya et al., [5] found lower sensitivity of 51.4% and higher specificity 81% than that we reported regarding MPV.
Conclusion
Platelet indices are not useful for diagnosis of sepsis. Platelet indices are not useful for prediction of mortality among critically ill children. Serum albumin and mechanical ventilation are more useful than platelet indices for prediction of mortality. Despite their failure to show association with sepsis diagnosis or mortality, Platelet indices were not completely devoid of diagnostic and prognostic values since they were significantly correlated with pSOFA score and some routine biomarkers like platelet count, ANC, and lymphocytic count.
Limitations of the present study include the small sample size. Further studies with a larger sample size are needed to properly assess the diagnostic and prognostic role of platelets indices among critically ill children since our current findings still show correlations between PLT indices and PRISM score as well as other biomarkers.
Further studies are required to establish the values of PLT indices helping to further stratify patients into risk of mortality, and thereby tailoring care to individual case-based level.
Author Statements
Acknowledgments
The authors would like to express their gratitude to the participants who participated in the study and the data collecting team.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Disclosure
The authors declare no conflicts of interest related to this work.
Funding
This research did not receive any fund.
Informed Consent
Consent was obtained from the parents (or legal representative).
Ethical Approval
All procedures performed in the study were in accordance with the ethical standards of the Menoufia University and Faculty of Medicine.
Author Contributions: Conceptualization, Nagwan Saleh, Ahmed Khatab; Formal analysis, Nagwan Saleh and Muhammad El- Mekkawy; Funding acquisition, Nagwan Saleh, Ahmed Khatab, Muhammad El- Mekkawy, and Shaimaa Nomair; Investigation, Shaimaa Nomair , Nagwan Saleh; Methodology, Nagwan Saleh, Ahmed Khatab, Muhammad El- Mekkawy, and Shaimaa Nomair; Resources, Nagwan Saleh, Ahmed Khatab, Muhammad El- Mekkawy, and Shaimaa Nomair; Supervision, Nagwan Saleh, Ahmed Khatab, Muhammad El- Mekkawy, and Shaimaa Nomair; Writing – review & editing, Nagwan Saleh. All authors have read and agreed to the published version of the manuscript.
References
- Pinto NP, Rhinesmith EW, Kim TY, Ladner PH, Pollack MM. Long- term function after Pediatric Critical illness: Results from the survivor outcomes study. Pediatric Critical Care Medicine. 2017; 18: 122–30.
- Simonis FD, Neto AS, Binnekade JM, Braber A, Bruin KC, Determann RM. Effect of a low Vs intermediate tidal volume strategy on ventilator-free days in intensive care unit patients without ARDS: a randomized clinical trial. JAMA. 2018; 320: 1872-80.
- Bassetti M, Garnacho-Montero J, Calandra T, Kullberg B, Dimopoulos G, Azoulay E. Intensive care medicine research agenda on invasive fungal infection in critically ill patients. Intensive Care Medicine. 2017; 43: 1225-38.
- Herrup EA, Wieczorek B, Kudchadkar SR. Characteristics of postintensive care syndrome in survivors of pediatric critical illness: A systematic review. World Journal of Critical Care Medicine. 2017; 6: 124.
- Purbiya P, Golwala ZM, Manchanda A, Sreenivas V, Puliyel JM. Platelet Distribution Width to Platelet Count Ratio as an Index of Severity of Illness. The Indian Journal of Pediatrics. 2018; 85: 10–4.
- Djordjevic D, Rondovic G, Surbatovic M, Stanojevic I, Stanojevic I, Udovicic I, et al. Neutrophil-to-lymphocyte ratio, monocyte-to-lymphocyte ratio, platelet-to-lymphocyte ratio, and mean platelet volume-to-platelet count ratio as biomarkers in critically ill and injured patients: which ratio to choose to predict outcome and nature of bacteremia?. Mediators of Inflammation. 2018; 2018: 3758068.
- Noris P, Melazzini F, Balduini CL. New roles for mean platelet volume measurement in the clinical practice? Platelets. 2016; 27: 607–12.
- Golwala ZM, Shah H, Gupta N, Sreenivas V, Puliyel JM. Mean Platelet Volume (MPV), Platelet Distribution Width (PDW), platelet count and Plateletcrit (PCT) as predictors of in-hospital pediatric mortality: a case-control study. African Health Sciences. 2016; 16: 356–62.
- Sayed SZ, Mahmoud MM, Moness HM, Mousa SO. Admission platelet count and indices as predictors of outcome in children with severe Sepsis: a prospective hospital-based study. BMC pediatrics. 2020; 20: 1–9.
- Goldstein B, Giroir B, Randolph A. International pediatric sepsis consensus conference: definitions for sepsis and organ dysfunction in pediatrics. Pediatr Crit Care Med. 2005; 6: 2–8.
- Pollack MM, Ruttimann UE, Getson PR. Pediatric Risk of Mortality score. Crit Care Med. 1988; 16: 1110–6.
- Slater A, Shann F, Pearson G. PIM 2: A revised version of the Pediatric Index of Mortality. Intensive Care Med. 2003; 29: 278–85.
- Schlapbach LJ, Straney L, Bellomo R, MacLaren G, Pilcher D. Prognostic accuracy of age-adapted SOFA, SIRS, PELOD-2, and qSOFA for in-hospital mortality among children with suspected infection admitted to the intensive care unit. Intensive Care Med. 2018; 44: 179–88.
- Guclu E, Durmaz Y, Karabay O. Effect of severe sepsis on platelet Count and their indices. African Health Sciences. 2013; 13: 333–8.
- Zhang Z, Xu X, Ni H, Deng H. Platelet indices are novel predictors of hospital mortality in intensive care unit patients. Journal of Critical Care. 2014; 29: 885.e1-6.
- Abd-Abd-Elmoneim A, Alhojaili AR, Banjer NR, Zolaly M, Abd El-Moneim ES. Changes in mean platelet volume in critically ill children and its relation to other clinical and laboratory findings. 2018; 5: 29–35.
- Leile J, Borne AK. Increased mean platelet volume in septicaemia. Journal of Clinical Pathology. 1983; 36: 693–6.
- Fioretto M Jr, Jg K, Cs C, Mf B, Rc de M, M.a. Comparison between procalcitonin and C-reactive protein for early diagnosis of children with sepsis or septic shock. Inflammation Research. 2010; 59: 581–6.
- Fioretto M Jr, Jg K, Cs C, Mf B, Rc de M, M.a. Comparison between procalcitonin and C-reactive protein for early diagnosis of children with sepsis or septic shock. Inflammation Research. 2010; 59: 581–6.
- Becker KL, Snider R, Nylen ES. Procalcitonin assay in systemic inflammation, infection, and sepsis: clinical utility and limitations. Critical Care Medicine. 2008; 36: 941–52.
- Tang BM, Eslick GD, Craig JC, McLean AS. Accuracy of procalcitonin for sepsis diagnosis in critically ill patients: systematic review and meta-analysis. The Lancet Infectious Diseases. 2007; 7: 210–7.
- Farag NA, Taema KM, Abdel-Latiff E, Hamed G. Differentiating sepsis from non-infective systemic inflammatory response syndrome: comparison between C-reactive protein and Leptin. The Egyptian Journal of Critical Care Medicine. 2013; 1: 111–8.
- Sriram SM, Aroor S, Kini PG, Kanaparthi S, Konda K. Platelet indices in children with sepsis and their relation to the outcome. Sri Lanka Journal of Child Health. 2018; 47: 301–5.
- Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. New England Journal of Medicine. 2003; 348: 1546-54.
- Vardon-Bounes F, Gratacap MP, Groyer S, Ruiz S, Georges B, Seguin T. Kinetics of mean platelet volume predicts mortality in patients with septic shock. PLOS One. 2019; 14: e0223553.
- Choi SJ, Ha EJ, Jhang WK, Park SJ. Platelet indices as predictive markers of prognosis in pediatric septic shock patients. mortality. 2017; 21: 25–3.
- Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Sm O. Campaña para sobrevivir a la sepsis: recomendaciones internacionales para el tratamiento de sepsis grave y choque septicémico, 2012. CCMJ. 2013; 41: 36–44.
- El-Mashad GM, El-Mekkawy MS, Zayan MH. Paediatric Sequential Organ Failure Assessment (pSOFA) score: a new mortality prediction score in the paediatric intensive care unit. Anales de Pediatría. 2020; 92: 277–85.
- Vanderschueren S, Weerdt A, Malbrain M, Vankersschaever D, Frans E, Wilmer A. Thrombocytopenia and prognosis in intensive care. Critical Care Medicine. 2000; 28: 1871–6.
- Becchi C, Al Malyan M, Fabbri LP, Marsili M, Boddi V, Boncinelli S. Mean platelet volume trend in sepsis: is it a useful parameter? Minerva Anestesiologica. 2006; 72: 749–50.
- Patrick CH, Lazarchick J. The effect of bacteremia on automated platelet measurements in neonates. Am J Clin Pathol. 1990; 93: 391–4.
- Ye S, Zhang Y, Zhang C, Xu D. Are platelet volume indices related to mortality in hospitalized children on mechanical ventilation? Journal of International Medical Research. 2018; 46: 1197–208.
- Girish N, Srinivas HA, Soumya A. A study of hypoalbuminemia in critically ill children in pediatric intensive care unit. International Journal of Contemporary Medicine. 2016; 4: 44–8.
- Ely EW, Baker AM, Dunagan DP, Burke HL, Smith AC, Pt K. Effect on the duration of mechanical ventilation of identifying patients capable of breathing spontaneously. New England Journal of Medicine. 1996; 335: 1864–9.
- Epstein D, Khemani R, Wong C, Waters K, Kipke M, Markovitz B. Health insurance type, not race, affects children’s mortality in the pediatric intensive care unit (PICU. Critical Care Medicine. 2009; 37: 11–2.
- Kim CH, Kim SJ, Lee MJ, Kwon YE, Kim YL, Park KS. An increase in mean platelet volume from baseline is associated with mortality in patients with severe sepsis or septic shock. PLOS one. 2015; 10.
- Gao Y, Li Y, Yu X, Guo S, Ji X, Sun T. The impact of various platelet indices as prognostic markers of septic shock. PLOS One. 2014; 9.