
Review Article
Austin J Clin Med. 2024; 9(1): 1048.
Traditional Chinese Medicine Regulates Gut Microbiota: A Possible Mechanism for TCM’s Anti-Hepatic
Khair Ullah*
Guangzhou Institutes of Biomedicine and Health (GIBH) 190 Kaiyuan Blvd, Guangzhou, GUANGDONG, China
*Corresponding author: Khair Ullah Guangzhou Institutes of Biomedicine and Health (GIBH) 190 Kaiyuan Blvd, Guangzhou, Guangdong, China. Email: khair@gibh.ac.cn
Received: June 17, 2024 Accepted: July 10, 2024 Published: July 17, 2024
Abstract
Many diseases, including liver fibrosis, have a direct connection to gut dysbiosis. The liver, which interacts with the intestinal tract most closely, is exposed to the gut microbiome or their metabolites. An altered gut microbiome was found to be associated with several liver complications, including liver fibrosis. Age, diet, environment, use of antibiotics, and stress can all change the Gut Microbiota’s (GM) composition. The severity of liver steatosis, inflammation, and fibrosis may be influenced by this dysbiosis through a variety of interactions with the host’s immune system and other cell types. This review explores the TCM formulations that regulate GM as a potential anti-fibrotic mechanism. Herein, we comprehensively reviewed the basic research from the last 20 years and found that herbal TCMs (including hawthorn, Si-Wu-Tang, ganshuang granules, yinchenhao decoction, sanwei ganjiang powder, and huang qi decoction) have potential clinical efficacy against liver fibrosis by gradually exerting the regulatory effect on GM. The significant TCM activities include regulating immunity, lowering serum ammonia levels, enhancing lipid metabolism, promoting intestinal barrier integrity and function, and overcoming fibrogenesis of the liver. The underlined functions are all highly correlated to the TLR4 signaling cascade and involve ROS, NF-κB, RhoA/ROCK1, and NOX4/ROS. Hence, the underlined herbal TCMs can serve as drug candidates for liver fibrosis therapy by supplementing probiotics and adjusting the balance of GM. However, the underlying complex molecular mechanism of TCM’s-regulated GM nexus in liver fibrosis still needs to be explored further.
Keywords: Decoctions; Gut dysbiosis; Gut Microbiota (GM); Inflammation; Liver fibrosis; Traditional Chinese Medicine (TCM)
Introduction
Since ancient times, Traditional Chinese Medicine (TCM) has been used to treat patients with a wide range of ailments in China and is currently extended to use worldwide [1]. Herbal TCM is the main pharmacological therapy of TCM. Even during the COVID-19 coronavirus pandemic, TCM and Western medications were employed to contain and ultimately overcome the disease's spread [2]. Herbal TCM can improve the clinical symptoms of various complications, reverse some pathological changes and maintain the body’s normal physiological function [3]. Numerous disorders are mostly treated with Western medicinal interventions because Western medicine was introduced to China in the 16th century C.E. TCM has gradually transformed from conventional therapy toward alternative therapy [4]. However, there are still some medical conditions where TCM therapy is beneficial, such as liver diseases, where Western medication has not been as effective [5].
This review highlighted the influence of GM on liver fibrosis and focused on the role of TCM herbal formulations against hepatic fibrosis by regulating GM. Since herbal TCMs (including hawthorn, Si-Wu-Tang, ganshuang granules, yinchenhao decoction, sanwei ganjiang powder, and huang qi decoction) have potential clinical efficacy against liver fibrosis by gradually exerting the regulatory effect on GM. The finding that human GM significantly affects metabolism has opened a new paradigm in modern therapeutic approaches. The balance of the GM, which is linked to intestinal health as well as the liver, brain, kidneys, and organismal homeostasis, affects the processes of birth, aging, disease, and death [6,7]. The GM is an integral bodily part that functions in many ways as an additional organ [8]. In terms of the Chinese approach to the “holistic concept, gut microbes are significant to TCMs. Several studies have demonstrated that GM may play a part in a variety of human disorders, such as Inflammatory Bowel Disease (IBD), liver fibrosis, Nonalcoholic Fatty Liver Disease (NAFLD), obesity, and diabetes [9-13]. Additionally, numerous interactions between the chemical elements of TCMs and the GM are currently the subject of future investigations [14,15]. To be more specific, the GM can either metabolize TCM components on its own or co-metabolize chemicals with the host. The metabolites that are made have different levels of bioavailability, bioactivity, and toxicity. Components of TCM can also modulate the composition of the GM to aid in homeostasis recovery. TCM can thereby improve relevant clinical diseases as well as GM dysfunction. According to the underlined findings, GM can mediate either synergistic or antagonistic effects among various TCM components [16]. GM is also being studied by researchers as a starting point for the investigation of biomarkers and treatments for liver disorders outside of the gut. The "holistic concept of TCM" is consistent with this study. The fact that GM and its metabolites play a role in the development of liver diseases suggests that TCM could be used to treat liver diseases by regulating gut flora [17]. Therefore, we believe that TCM has a role in the regulation of GM which is the possible mechanism of the TCM’s anti-hepatic fibrosis activity. Herein, we reviewed the basic research from the past two decades to evaluate the most effective TCMs associated with GM regulation and anti-hepatic fibrosis effects and to provide new evidence for its clinical application.
Methods
Literature Search Strategy
For this study, Google scholar, PubMed, Embase, and CNKI databases were searched for the articles that were published between January 2000 and December 2022. The keywords included "herbal traditional Chinese medicine", "liver fibrosis", "anti-fibrotic mechanism", "gut microbiota", "gut dysbiosis", "TCM decoctions", "cognitive impairment", and their related terms. A total of 1695 articles were identified.
Study Selection
The following inclusion criteria were used for screening of the selected articles: The articles that have revealed herbal TCMs' antifibrotic effect, TCM formulations, and decoctions associated with antifibrotic activities or in the regulation of gut microbiota. The 1695 articles identified by the search engine were then manually screened for those that met our inclusion criteria based on title and abstract. This led to the exclusion of 1025 articles due to duplication, irrelevance, lack of abstract, or unavailability of full text while the remaining 670 articles, including 291 basic studies, 215 clinical studies, and 164 reviews or meta-analyses were included. There was a total of 276 papers that constituted the underlying research. The 29 studies (revealing some potential herbal TCM formulations/decoctions with potent anti-hepatic liver fibrosis effect) were selected from the basic and clinical studies.
Data Extraction
Two authors have independently studied the titles, abstracts, and full texts of the retrieved articles. As a result, 260 articles were finally included, followed by data collection based on the predetermined criteria. Any disagreements were resolved through discussion among the authors.
Liver fibrosis and GM
The liver's response to injury is the scarring process known as hepatic fibrosis. The liver repairs damage through the deposition of new collagen, much like how the skin and other organs heal wounds by depositing collagen and other matrix components [18]. Most chronic liver illnesses are linked to hepatic fibrosis, a prevalent and possibly fatal consequence that places a heavy financial and medical burden on society. The development of hepatic fibrosis is not a straightforward process; rather, it involves the interaction of several soluble mediators (cytokines and chemokines) with various cellular subsets that exist in and affects the liver. The chemical and biological characteristics of the disease-causing substances further modulate liver fibrosis [19]. Liver fibrosis is the outcome of the liver's attempt to heal damage from a variety of damaging triggers, such as genetic disorders, long-term viral infections, alcoholism, autoimmune diseases, metabolic abnormalities, cholestasis, venous blockage, and parasite infections (Figure 1) [20]. The excessive deposition of Extracellular Matrix (ECM) components and dysfunction of Liver Sinusoidal Endothelial Cells (LSECs) are the primary causes of liver fibrosis. Under physiological circumstances, LSECs act as the gatekeepers of a steady liver environment [21]. The LSECs' anti-fibrotic properties enable hepatic vascular resistance regulation and lower venous pressure by preventing Kupffer cells from activating and the development of Hepatic Stellate Cells (HSCs). These LSECs' endothelial basement membrane capillary vascularization, which takes place in the early phases of liver fibrosis, creates several pathogenic variables and alters liver function [22,23]. The onset and progression of liver disease can also be caused by problems with GM. This process can eventually lead to liver cirrhosis, a condition in which the architectural arrangement of the liver's functional units is so disrupted that blood flow through the liver and liver function are both compromised [24]. Serious liver disease consequences, such as portal hypertension, liver failure, and liver cancer, may manifest once cirrhosis has been established. Cirrhosis should be regarded as a pre-malignant condition because it markedly raises the chances of liver cancer once it progresses [25].
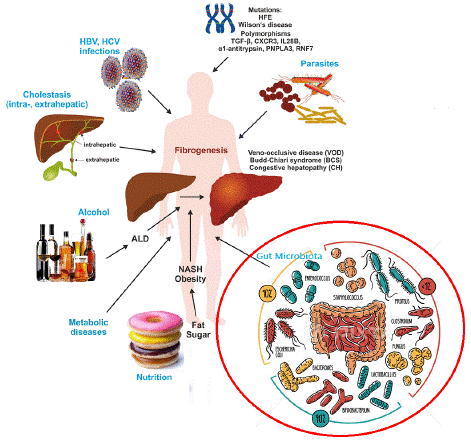
Figure 1: An illustration of the major causes of hepatic fibrosis. Inherent genetic mutations and disorders in population is one of the fundamental factor ultimately contributing to liver fibrosis. Parasitic and infectious diseases like hepatitis B and C constitutes pathophysiological plethora exerting fibrogenic lesions in liver. Cholestasis associated complications disturbs the fatty metabolism which can lead to liver fibrosis. Lifestyle factors, obesity, and fatty liver disease constitutes the major causes of steatosis in liver. While finally the gut microbiota dysbiosis is one of the emerging factor contributing towards the development fibrosis in liver.
In terms of structure and functionality, the gut and the liver are closely related organs; the intestine supplies 75% of the blood that enters the liver's portal vein [26]. The liver and intestine can interact with one another through a variety of physiological processes since the liver-gut axis is present. GM has a significant impact on pathophysiological alterations in the liver [27,28].
As a virtual organ, the gastrointestinal tract forms connections with a number of extraintestinal organs, including the brain, liver, and kidney, as well as the cardiovascular and endocrine systems. It is an essential component in the production of many metabolites, such as bile acids, which control various metabolic pathways in the host. It is the primary modulator of host metabolism; it makes it easier to extract nutrients and energy from food consumed and helps the host use those nutrients and energy more efficiently [29,30]. The gut-liver axis's ability to work properly depends heavily on the integrity of the intestinal barrier. When intestinal antigens (IAg, which come from food or pathogenic bacteria) enter the portal circulation, they are recognized by T Cell Receptors (TCRs) and consequently cause the adaptive immune system to become activated.
A complex network of factors, including cytokines, immunological cells, and IAg, regulates and stabilizes the relationship between the GM and the intrahepatic immunological microenvironment. GM dysbiosis causes resident liver macrophages (i.e., Kupffer cells) to become activated, releasing pro-inflammatory cytokines (such as IL-6) and stimulating HSCs to produce matrix through Toll-Like Receptors (TLRs) [31,32]. Additionally, excessive production of bacterial fragments and products, such as peptidoglycans, lipopolysaccharides, and flagellin, enter the liver through the portal [33]. In individuals with pre-cirrhotic liver disease and cirrhosis, there is emerging evidence that the GM alters after intestinal microbiota transplantation. Bacteria in the blood increase in response to hepatic fibrogenesis, suggesting that the GM affects how quickly liver fibrosis progresses. Furthermore, numerous investigations have shown a correlation between GM composition, alcohol, and ROS production [34-37].
Additionally, the GM may impact and regulate liver regeneration. After partial hepatectomy, the number of CD1d-dependent Natural Killer T (NKT) cells was found to be much lower after being treated with antibiotics, especially ampicillin. An elevated level of interferon-γ and IL-12 cytokines released by these NKT cells and activated Kupffer cells eliminated the adverse effects of antibiotic treatment on liver regeneration. Therefore, antibiotic treatment following hepatectomy may adversely affect liver regeneration in humans [38]. According to recent studies, some gut bacteria are strongly correlated to the production of a few genes in a regenerated liver [39]. More than 6000 bacterial microbiota-related genes were upregulated as a result of partial hepatectomy, some of which were implicated in bile acid metabolism and hepatocyte proliferation. The IM had significant alterations as a result of this surgery, such as a rise in Bacteroidetes and Rikenellaceae and a decrease in Clostridiales, Lachnospiraceae, and Ruminococcaceae [39]. Wnt factors and traditional inflammatory cytokines are well known to regulate liver regeneration and tissue repair [40,41]. Although sterile inflammation might be the main factor at play, in this case, it's also possible that GM acts as another major factor in such inflammation. The gut-liver axis's ability to work properly largely depends on the integrity of the intestinal barrier. As a result, the GM appears to be an underutilized player for effective liver regeneration.
The Gut-Liver Axis
The bidirectional interaction between the gut and its microbiota and the liver, which is the result of the integration of signals produced by nutritional, genetic, and environmental variables, is referred to as the "gut-liver axis" [42]. The human body's liver and gut are connected anatomically and physiologically (Figure 2) [43]. The functional and physical structures referred to as the intestinal mucosal and vascular barriers serve as a site for interactions between the gut and the liver, preventing the systemic spread of pathogens and toxins while permitting nutrients to enter the bloodstream and reach the liver [44]. The liver influences GM communities as part of this bidirectional connectivity, which is essential for maintaining the homeostasis of the gut-liver axis [45]. Moreover, it was discovered that the GM of rats with hepatic fibrosis was altered [46], and a subsequent study demonstrated that liver damage and liver fibrosis are eventually caused by intestinal barrier malfunction and the leakage of intestinal bacteria into the blood [47]. These alterations also lower the levels of bacterial metabolites, including lysozyme and lysophosphatide that might cause liver fibrosis and prevent Kupffer cell activation [48].
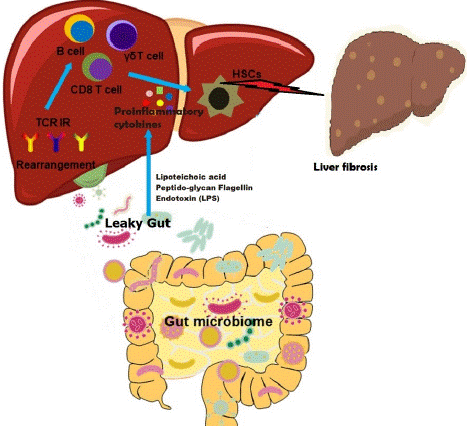
Figure 2: The impact of the gut microbiome on the intrahepatic immune microenvironment in liver fibrosis through the gut-liver axis. The gut microbiome dysbiosis leads to leaky gut and release of endotoxins (lipoteichoic acid, peptide-glycan flagellin, and lipopolysaccharide) and proinflammatory milieu surge in hepatic circulation. The release of inflammatory cytokines is associated with T cells, monocytes, and resident macrophages which in turn promote inflammation and fibrotic matrix growth while affecting hepatic stellate cells (HSCs).
The gut-liver axis uses a two-way regulatory mechanism, meaning that both liver fibrosis and the intestinal microbiome can influence one another [49]. When liver fibrosis is caused by chronic hepatitis B, the number of intestinal microbiota decreases, which changes the way bile acids, is processed [50]. In addition to increasing the quantity of Lactobacillus and Bifidobacterium in the flora [51,52], ursolic acid can inhibit liver fibrogenesis and promote the stability of the bacterial flora [52]. The composition of the GM has also been linked to inflammation and liver fibrosis [53], and supplementation with the probiotic L. rhamnosus has been found to reduce liver fibrosis and inflammation [54].
TCM formulations with Significant Anti-Fibrotic Effects Regulate the GM System
Hawthorn (HAW, Shan Zha)
The Chinese herb, hawthorn berry also known as Shan Zha. Vitexin, quercitin, oleanolic acid of triterpenes, ursolic acid, chlorogenic acid of organic acids, and maslinic acid are the key constituents of HAW [56,57]. By regulating oxidative stress and inflammation, the HAW decoction from Crataegus oxyacantha suppresses the fibrotic effects of CCL4. According to the reported studies, the anti-fibrotic potential of HAW is due to its chemical constituents, i.e., quercetin, vitexin, and maslinic acid [58]. By inhibiting inflammasome response and reticulum stress pathway activation, quercetin can reverse GM imbalance and TLR-4 pathway inductions mediated by endotoxemia. This prevents the deregulation of gene expression and blocks lipid metabolism, which overcomes NAFLD in HFD mice and reduces hepatic damage, including hepatic fibrosis [59]. Quercetin, therefore, exerts its anti-inflammatory effects via integrative reactions involving GM dysbiosis, associated gut-liver axis activation and lipotoxicity blockade, followed by suppression of inflammasome response and activation of the reticulum stress pathway [60]. Another constituent is vitexin, a naturally occurring flavonoid molecule with a variety of pharmacological properties, including the ability to modulate lipid metabolism and reduce inflammation. Vitexin can increase fatty acid oxidation and lipolysis in addition to suppressing de-novo lipogenesis. It can also improve insulin signalling in HFD mice by activating AMPK and interacting with the leptin receptor [61,62]. Additionally, by blocking TLR4/NF-B signalling and the production of proteins involved in the fatty acid synthesis, vitexin may reduce the effects of chronic stress [63]. It is a pentacyclic triterpenoid called maslinic acid. Maslinic acid can decrease lipogenesis by activating AMPK in HepG2 cells, but it cannot decrease lipid accumulation in L02 cells treated with FFA by suppressing sterol regulatory element-binding protein cleavage-activating protein (SCAP) [64]. Maslinic acid has been shown to prevent hepatic steatosis in obese mice produced by the HFD via regulating the Sirt1/AMPK signalling cascades [65,66]. Additionally, HAW peel polyphenols exhibit potent activity against oxidative stress, which not only regulates Nrf-2/ARE expression but also affects liver MDA levels, T-SOD, and GSH-Px activities [67]. From the underlined studies on HAW, we concluded that HAW anti-fibrotic activity and restoring the healthy liver characteristics of animals induced by hepatic fibrosis is mechanistically underlying by the crosstalk between liver damage, and oxidative stress, inflammation, and activation of fibrogenesis. Furthermore, it showed that HAW works by reducing inflammation, controlling oxidative stress, and inhibiting HSC activation (Figure 3) [68].
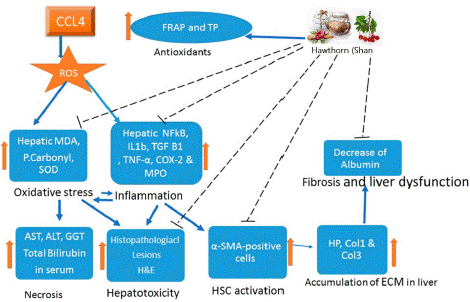
Figure 3: Hawthorn (HAW) exerts anti-inflammatory and antifibrotic effects in CCL4-induced hepatic fibrosis by inhibiting the pro-oxidant machinery and elevating the antioxidant effects [68]. The hepatic fibrosis induced by CCL4 is mediated by oxidative stress and inflammatory response leading to cellular damage, activation of hepatic stellate cells (HSCs), and accumulation of extracellular matrix (ECM) leading to liver fibrosis. HAW is shown to have positive effects on oxidative stress and inflammation; it suppresses HSCs activation and thereby relieves the progression of liver fibrosis.
Si-Wu-Tang (SWT)
TCM prescription Si-Wu Tang (SWT), which dates back to the Song Dynasty, was originally documented in Taiping Huimin Heji Jufang. SWT is comprised of Radix Angelica Sinensis, Radix Rehmanniae Praeparata, Radix Paeoniae Alba, and Rhizoma Ligusticum Chuanxiong [69]. The ECM deposition in livers, intestinal barrier dysfunction, and the hepatic and intestinal inflammatory responses were all considerably reduced when SWT was used to treat CCl4-induced liver fibrosis [70]. Notably, unconjugated serum Bile Acids (BAs) including Chenodeoxycholic Acid (CDCA) were greatly elevated in serum following SWT delivery, whereas conjugated BAs such as taurocholic acid (TCA) were significantly decreased, suggesting that SWT may change the makeup of BAs in circulation by controlling GM (Figure 4) [70,71]. These results further demonstrated that FXR-FGF15 enterohepatic and FXR-SHP hepatic pathways were responsible for SWT's effective inhibition of BA de-novo synthesis and enhancement of BA excretion, which also altered enterohepatic BA circulation and alleviated liver injury [70]. These findings emphasised the possible contribution that the gut-liver axis associated with BA may have to the beneficial effect of SWT. Taken together, these data revealed that SWT reduced liver fibrosis via modifying GM, which in turn changed the pathogenic BA profile and bacteria-associated intestinal and hepatic inflammation. Furthermore, the underlined research provides solid evidence for the therapeutic potential of SWT in the clinical management of fibrotic liver damage [70].
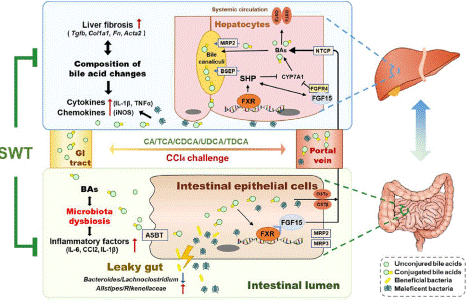
Figure 4: Si-Wu-Tang (SWT) overcomes the effect of hepatic fibrosis by regulating GM and bile acid levels [70]. This figure showed that dministrating SWT results in unconjugated bile acids (BAs) in serum significant elevation while conjugated bile acids (CBAs) tend to decrease. The data suggests that SWT may alter the composition of BAs in circulation by regulating gut microbiota (GM). Furthermore, SWT efficiently decreased the level of BA in de-novo synthesis and enhanced BA excretion mediated by FXR-FGF15 enterohepatic and FXR-SHP hepatic pathways, which also modulated enterohepatic BA circulation and relieving liver injury.
Ganshuang Granules (GSG)
Ganshuang granules (GSG) are an effective TCM prescription against liver fibrosis. GSG is comprised of Angelica sinensis, Nasturtium officinale, Codonopsis pilosula, Paeonia lactiflora, Atractylodes macrocephala, Wolfiporia extensa, Salvia miltiorrhiza, Hedgehog, Giant knotweed, Dandelion, Turtle shell, Selfheal, and Peach kernel [71].
According to the studies, GSG therapy reduced liver damage in CCl4-induced hepatic fibrosis as evidenced by a decrease in liver index, alanine aminotransferase levels, and aspartate transaminase levels. GSG reduced the CCl4-induced models' increased oxidative stress, inflammatory response, and hepatic fibrosis [72,73]. Furthermore, it was discovered that GSG increased the expression of tight junction-related proteins in the intestinal mucosa. Additionally, GSG corrected gut dysbiosis by reducing the ratio of Firmicutes to Bacterioidetes, increasing the alpha and beta diversity of GM, and adjusting the relative abundance of different bacteria. Finally, GSG attenuated CCl4-induced hepatic fibrosis by lowering intestinal permeability and rebalancing the GM to minimize oxidative stress and inflammation. Furthermore, several studies revealed that the treatment of chronic hepatitis B induced liver fibrosis with GSG and antiviral medication. A recent study found that GSG blocks the mammalian target of rapamycin, which stimulates liver stellate cells [74]. Another study suggested that GSG's anti-liver fibrosis mechanism involves inhibiting regulatory T cells [75].
Yinchenhao Decoction (YCHD)
Since the Han Dynasty, Yinchenhao Decoction (YCHD) has been a traditional TCM herbal remedy for treating the damp-heat syndrome of the gallbladder and liver [76]. YCHD is formulated from Gardenia jasminoides J. Ellis, Artemisia capillaries Thunb, and Dahuang. It has been used to cure liver disorders, including liver fibrosis, for more than 1800 years, making it one of the most important medicines [77,78]. Several studies have reported different mechanisms for the role of YCHD in liver diseases such as liver fibrosis. A recent study found that the use of YCHD improves metabolism and increases the abundance of GM like Bacteroidetes, Bifidobacterium, Lactobacillus, and Bacteroidetes/Firmicutes. Dysbiosis of the underlined microbiota leads to the activation of resident liver macrophages releasing proinflammatory cytokines, stimulation of matrix synthesis by HSCs through TLRs [79], excessive production of microbial fragments and products (including peptidoglycans, flagellin, and LPS), which get into the liver through the portal system and can cause liver fibrosis and long-term inflammation. According to another study, YCHD's anti-fibrotic effects may be linked to the downregulation of TGF-1 and the restoration and rebuilding of the Renin-Angiotensin System's (RAS) own self-regulation by increasing the protein production of Angiotensin-II (ACE2). YCHD considerably reduced the amount of collagen in liver tissue and markedly enhanced liver function in a rat model of DMN-induced liver fibrosis [80]. YCHD may help treat liver fibrosis by lowering oxidative stress and the lipid peroxidation that comes with it, inhibiting HSC proliferation, and activating HSCs through the TGF-1/Smad/ERK signalling cascade. Chronic hepatitis B, which is the principal cause of liver fibrosis, can also be treated with YCHD [81]. Taken together, YCHD can target several pathways associated with liver diseases.
Sanwei Ganjiang Powder (SWGJ)
The Jia Ga Song Tang, commonly known as Sanwei Ganjiang Powder (SWGJ), is made up of Alpinia katsumadai, Myristica fragrans Houtt, and Zingiberis Rhizoma in a ratio of 5:4:6 accordingly. In mice with acute hepatic injury, SWGJ had the impact of modulating intestinal flora and associated immune cytokines [82]. SWGJ reduced liver damage, and this action was correlated with modulating GM to balance bile acid homeostasis. They have anti-inflammatory effects, protect the digestive tract's mucosa, aid in the defense against oxidation, and shield the liver. Following the injection of SWGJ, proteins involved in the production (i.e., CYP7A1), excretion, and reabsorption of bile acids (such as NTCP, Mrp2, and BESP) were increased in a CCl4-induced model of chronic liver injury [82]. According to another study, SWGJ protects the liver through the Nrf2-ARE pathway [83–85]. The underlined results showed that SWGJ had protective effects against liver and intestine damage, and activation of Nrf2 was a potential mechanism for improving bile acid enterohepatic circulation. Consequently, SWGJ may be a possible therapeutic approach for preserving the gut-liver axis. Moreover, SWGJ regulates GM in addition to protecting the liver [87] based on the gut-liver axis [85,86].
Largehead Atractylodes Rhizome (LAR)
Largehead atractylodes rhizome (LAR) is an important herbal TCM comprised of volatile oils (humu-lene, β-elemol, a-curcumene, atractlone, 3β-acetoxyatractylone), Sesquiterpene lactone compounds (atractylenolide, 8β-ethoxyatractylenolideII), and polyacetylene (14- acetyl-12-senecioyl2E,8Z,10E-atracetylentriol) which invigorate the spleen and replenish qi (life force/ vital energy). LAR eases the symptoms of chronic diarrhoea, leg weakness, gastro and abdominal distention, and indigestion [88]. According to the reported studies, LAR plays an effective role in the treatment of liver fibrosis and liver cancer [89]. Moreover, it has a key role in the regulation of GM which has a significant link with liver fibrosis [90]. Pathogenic bacteria, including H. pylori and Clostridium, are much less prevalent after LAR treatment. However, there is a considerable elevation in beneficial bacteria such as Lactobacillus and Akerman's bacteria, suggesting that LAR stimulates probiotic proliferation and keeps the gut flora in a balanced state [91].
The control of E. coli, Shigella, and Alternaria, as well as the homeostasis of the GM, is associated with the therapeutic benefits of LAR in vivo [92]. H. pylori infection may make inflammatory lesions in the stomach worse in people with liver fibrosis, which may directly or indirectly affect liver function [93]. While LAR can inhibit the proliferation of H. pylori and reduce the expression of the inflammatory factors. Furthermore, LAR has been shown to balance the internal environment, maintain GM, and treat chronic gastrointestinal disorders effectively [94]. Moreover, it inhibits liver cancer cell metastasis and promotes cellular immune function [95]. Hence, the underlined studies suggested that LAR may play a considerable role in the treatment of liver fibrosis by regulating GM and decreasing the level of inflammatory factors including IL-8.
Huangqi Decoction (HQD)
A classical herbal TCM formulation, Huangqi Decoction (HQD), is composed of Glycyrrhizae Radix et Rhizoma and Astragali Radix, in a ratio of 1:6 (wt/wt) [97]. Several studies have demonstrated the beneficial role of HQD against hepatic fibrogenesis [98-100]. Moreover, it has been confirmed that HQD protects against alpha-naphthylisothiocyanate-induced cholestatic liver injury [101]. HQD can significantly increase the diversity of GM in cholestatic mice and enhance the GM's overall composition. According to the reported studies, the protective effects of HQD on hepatic injury and fibrosis may be related to its role in GM regulation [97].
The liver serves as the first line of defence against intestinal enterobacteria or metabolites because of the anatomical connection known as the "gut-liver axis" [102]. According to certain reports, intestinal dysbiosis can further disrupt intestinal barrier integrity [103]. The immune microenvironment of the liver can be altered as a result of the microbiota or related compounds "leaking" into the liver from the intestine. This immune response triggers the release of inflammatory molecules that exacerbate hepatic injury [104]. In mice with cholestasis, HQD decreased the expression of NLRP3 and other inflammatory markers and decreased inflammation in the colon tissue. This suggests that its ability to prevent hepatic inflammation and liver damage may be due to its ability to reverse intestinal dysbiosis and barrier integrity dysfunction [105].
Additionally, it has been stated that GM enzymes selectively convert glycyrrhizic acid from Glycyrrhizae Radix et Rhizoma (a component of HQD decoction) into absorbable components [106]. Astragalus polysaccharide, an efficient extract of Astragalus membranaceus and another ingredient in HQD decoction, has been shown in another study to work as an inflammatory reaction inhibitor by lowering the expression of proinflammatory factors like IL-6 and TNF-a [107,108]. Additionally, astragalus polysaccharides can decrease the amount of Salmonella typhi in the body and boost the production of Lactobacillus and Bifidobacterium in the intestinal tract, which both decrease the likelihood of an inflammatory reaction [109]. Astragalus polysaccharides can have anti-inflammatory effects as they reduce the number of bacteria in the body [110]. Taken together, herbal therapy, including HQD treatment, reduced GM dysbiosis, improved intestinal barrier dysfunction, decreased liver inflammation, and protected against hepatic injury and liver fibrosis.
Pueraria lobata (P. lobata)
Pueraria lobata or P. lobata is one of the most important medicinal herbs used traditionally in China. It has been established that P. lobata, which is derived from the dried roots of leguminous plants, has therapeutic activity [111]. P. lobata has been used in TCM to treat drunkenness and hangovers in China because it is believed to purify the liver and lessen the symptoms that follow [112]. Additionally, prior research discovered that puerarin extracted from P. lobata reduced ECM deposition in rats with CCl4-induced hepatic fibrosis, which attenuated hepatofibrosis [113, 114]. Puerarin prevented the growth and activation of hepatocytes originating from the ECM in fibrotic rats by blocking the endogenous TGF-1/Smad cascade, including HSCs, fibroblasts, and Kupffer cells. The fibrinogenolytic action of puerarin therapy on the fibrotic process makes it a promising therapeutic choice for the management of hepatofibrosis [115]. P. Lobata is used to extract Pueraria polysaccharides, which can then be ingested by intestinal microbes to improve metabolism and ultimately have an impact on physiological functions [116]. This TCM can significantly enhance the characteristics of drugs and lessen their toxicity after being fermented and digested in the gut flora [117]. P. Lobata lowers the expression level of inflammatory factors, including IL-6 and TNF-a. Using P. Lobata, it was demonstrated that the expression of the underlined inflammatory factors following Bifidobacterium fermentation was found to be considerably lower compared to Pueraria without fermentation [118]. Puerarin has also been demonstrated to raise the expression of the anti-inflammatory factor IL-10 and decrease the expression of the pro-inflammatory factors IL-6 and monocyte chemoattractant protein-1 in the colon and small intestine of mice fed a high-fat diet [119]. Other research has shown that Pueraria can protect the liver by lowering the amount of endotoxin [120,121].
Conclusions
Herbal TCMs (including Shan Zha, SWT, GSG, YCHD, SWGJ, LAR, and huangqi decoction) have potential clinical efficacy against liver fibrosis by gradually exerting the regulatory effect on GM. The relationship between GM and the host is complicated. However, the mechanism by which TCM regulates GM and halts the progression of the liver-associated disease involves a decrease in oxidative stress and inflammatory injury, which control the immune system. This, in turn, leads to a decrease in serum ammonia levels, an improvement in lipid metabolism, the protection of the intestinal barrier, and a reversal of hepatic fibrosis, all of which are significantly correlated to the TLR4 signaling pathway and involve NF-κB, ROS, and NOX4/RO. Hence, the underlined herbal TCMs can serve as a drug candidate for liver fibrosis therapy by supplementing probiotics and adjusting the balance of GM. Thus, research on the interaction between TCM and GM is essential for a better understanding of the pharmacological effects of TCM on the human body and the link between GM and disease. Future research should focus on individual therapies and extensively elucidate how GM, metabolites, and the healing potency of TCM are correlated. Moreover, the synergistic or antagonistic influence of GM and TCM is essential, particularly for the evaluation of prospective therapeutic efficacy, which provides an extensive understanding of developing difficulties before clinical use.
References
- Wang J, Yin XG, Wen Y, Lu J, Zhang RY, Zhou SH, et al. MPLA-Adjuvanted Liposomes Encapsulating s-Trimer or RBD or S1, But Not s-ECD, Elicit Robust Neutralization against SARS-CoV-2 and Variants of Concern. Journal of medicinal chemistry. 2022; 65: 3563-74.
- Lyu M, Fan G, Xiao G, Wang T, Xu D, Gao J, et al. Traditional Chinese medicine in COVID-19. Acta Pharmaceutica Sinica B. 2021; 11: 3337-63.
- Marshall AC. Traditional Chinese medicine and clinical pharmacology. Springer International Publishing. 2020.
- Dong J. The relationship between traditional Chinese medicine and modern medicine. Evidence-Based Complementary and Alternative Medicine. 2013.
- Ma Z, Zhang B, Fan Y, Wang M, Kebebe D, Li J, et al. Traditional Chinese medicine combined with hepatic targeted drug delivery systems: a new strategy for the treatment of liver diseases. Biomedicine & Pharmacotherapy. 2019; 117: 109128.
- Hills Jr RD, Pontefract BA, Mishcon HR, Black CA, Sutton SC, Theberge CR. Gut Microbiome: Profound Implications for Diet and Disease. Kompass Nutrition & Dietetics. 2022; 2: 3-18.
- Sharma R. Emerging Interrelationship Between the Gut Microbiome and Cellular Senescence in the Context of Aging and Disease: Perspectives and Therapeutic Opportunities. Probiotics and Antimicrobial Proteins. 2022; 14: 1-6.
- Clemente JC, Ursell LK, Parfrey LW, Knight R. The impact of the gut microbiota on human health: an integrative view. Cell. 2012; 148: 1258-70.
- Feng D, Zhang H, Jiang X, Zou J, Li Q, Mai H, et al. Bisphenol A exposure induces gut microbiota dysbiosis and consequent activation of gut-liver axis leading to hepatic steatosis in CD-1 mice. Environmental Pollution. 2020; 265: 114880.
- Manichanh C, Borruel N, Casellas F, Guarner F. The gut microbiota in IBD. Nature reviews Gastroenterology & hepatology. 2012; 9: 599-608.
- Cox AJ, West NP, Cripps AW. Obesity, inflammation, and the gut microbiota. The lancet Diabetes & endocrinology. 2015; 3: 207-15.
- Knip M, Siljander H. The role of the intestinal microbiota in type 1 diabetes mellitus. Nature Reviews Endocrinology. 2016; 12: 154-67.
- Qin J, Li Y, Cai Z, Li S, Zhu J, Zhang F, et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature. 2012; 490: 55-60.
- Li H, Zhou M, Zhao A, Jia W. Traditional Chinese medicine: balancing the gut ecosystem. Phytotherapy Research: An International Journal Devoted to Pharmacological and Toxicological Evaluation of Natural Product Derivatives. 2009; 23: 1332-5.
- Chen F, Wen Q, Jiang J, Li HL, Tan YF, Li YH, et al. Could the gut microbiota reconcile the oral bioavailability conundrum of traditional herbs?. Journal of Ethnopharmacology. 2016; 179: 253-64.
- Xu J, Chen HB, Li SL. Understanding the molecular mechanisms of the interplay between herbal medicines and gut microbiota. Medicinal Research Reviews. 2017; 37: 1140-85.
- Wang T, Huang S, Wu C, Wang N, Zhang R, Wang M, Mao D. Intestinal microbiota and liver diseases: insights into therapeutic use of traditional Chinese medicine. Evidence-Based Complementary and Alternative Medicine. 2021; 2021: 6682581.
- Bataller R, Brenner DA. Liver fibrosis. The Journal of clinical investigation. 2005; 115: 209-18.
- Weiskirchen R. Special Issue on “Cellular and Molecular Mechanisms Underlying the Pathogenesis of Hepatic Fibrosis”. Cells. 2020; 9: 1105.
- Tsochatzis EA, Bosch J, Burroughs AK. Liver cirrhosis. The Lancet. 2014; 383: 1749-61.
- Natarajan V, Harris EN, Kidambi S. SECs (sinusoidal endothelial cells), liver microenvironment, and fibrosis. BioMed research international. 2017; 2017: 4097205.
- Wilkinson AL, Qurashi M, Shetty S. The role of sinusoidal endothelial cells in the axis of inflammation and cancer within the liver. Frontiers in Physiology. 2020; 11: 990.
- Liu YT, Qi SL, Sun KW. Traditional Chinese medicine, liver fibrosis, intestinal flora: is there any connection?-a narrative review. Ann. Palliat. Med. 2021; 10: 4846-57.
- Riordan SM, Williams R. The intestinal flora and bacterial infection in cirrhosis. Journal of hepatology. 2006; 45: 744-57.
- Pinter M, Trauner M, Peck-Radosavljevic M, Sieghart W. Cancer and liver cirrhosis: implications on prognosis and management. ESMO open. 2016; 1: e000042.
- Hu B, Phan SH. Myofibroblasts. Current opinion in rheumatology. 2013; 25: 71.
- Palma P, Mihaljevic N, Hasenberg T, Keese M, Koeppel TA. Intestinal barrier dysfunction in developing liver cirrhosis: an in vivo analysis of bacterial translocation. Hepatology Research. 2007; 37: 6-12.
- Assimakopoulos SF, Tsamandas AC, Tsiaoussis GI, Karatza E, Triantos C, Vagianos CE, et al. Altered intestinal tight junctions’ expression in patients with liver cirrhosis: a pathogenetic mechanism of intestinal hyperpermeability. European journal of clinical investigation. 2012; 42: 439-46.
- Ramírez-Pérez O, Cruz-Ramón V, Chinchilla-López P, Méndez-Sánchez N. The role of the gut microbiota in bile acid metabolism. Annals of hepatology. 2018; 16: S21-S26.
- Liu Q, Liu S, Chen L, Zhao Z, Du S, Dong Q, et al. Role and effective therapeutic target of gut microbiota in NAFLD/NASH. Experimental and therapeutic medicine. 2019; 18: 1935-44.
- Zamparelli MS, Rocco A, Compare D, Nardone G. The gut microbiota: A new potential driving force in liver cirrhosis and hepatocellular carcinoma. United European Gastroenterology Journal. 2017; 5: 944-53.
- O’Rourke JM, Sagar VM, Shah T, Shetty S. Carcinogenesis on the background of liver fibrosis: Implications for the management of hepatocellular cancer. World journal of gastroenterology. 2018; 24: 4436.
- Zhou R, Fan X, Schnabl B. Role of the intestinal microbiome in liver fibrosis development and new treatment strategies. Translational Research. 2019; 209: 22-38.
- Ohtani N, Kawada N. Role of the gut–liver axis in liver inflammation, fibrosis, and cancer: a special focus on the gut microbiota relationship. Hepatology Communications. 2019; 3: 456-70.
- Bajaj JS, Khoruts A. Microbiota changes and intestinal microbiota transplantation in liver diseases and cirrhosis. J Hepatol. 2020; 72: 1003–1027.
- Tilg H, Cani PD, Mayer EA. Gut microbiome and liver diseases. Gut. 2016; 65: 2035-44.
- Weiskirchen R, Weiskirchen S, Tacke F. Recent advances in understanding liver fibrosis: bridging basic science and individualized treatment concepts. F1000Research. 2018; 7: F1000.
- Wu X, Sun R, Chen Y, Zheng X, Bai L, Lian Z, et al. Oral ampicillin inhibits liver regeneration by breaking hepatic innate immune tolerance normally maintained by gut commensal bacteria. Hepatology. 2015; 62: 253-64.
- Liu HX, Rocha CS, Dandekar S, Wan YJY. Functional analysis of the relationship between intestinal microbiota and the expression of hepatic genes and pathways during the course of liver regeneration. Journal of hepatology. 2016; 64: 641-50.
- Karin M, Clevers H. Reparative inflammation takes charge of tissue regeneration. Nature. 2016; 529: 307-15.
- Betrapally NS, Gillevet PM, Bajaj JS. Gut microbiome and liver disease. Translational Research. 2017; 179: 49-59.
- Jeon JW, Choi N, Lee SH, Sung JH. Three-tissue microphysiological system for studying inflammatory responses in gut-liver Axis. Biomedical Microdevices. 2020; 22: 65.
- Ghoshal UC, Shukla R, Ghoshal U. Small intestinal bacterial overgrowth and irritable bowel syndrome: a bridge between functional organic dichotomy. Gut and liver. 2017; 11: 196.
- Qin T, Fu J, Verkade HJ. The role of the gut microbiome in graft fibrosis after pediatric liver transplantation. Human Genetics. 2021; 140: 709-24.
- Ichinohe T, Pang IK, Kumamoto Y, Peaper DR, Ho JH, Murray TS, et al. Microbiota regulates immune defense against respiratory tract influenza A virus infection. Proceedings of the National Academy of Sciences. 2011; 108: 5354-9.
- Li Z, Ni M, Yu H, Wang L, Zhou X, Chen T, Liu G, Gao Y. Gut microbiota and liver fibrosis: One potential biomarker for predicting liver fibrosis. BioMed Research International. 2020; 2020: 3905130.
- Chopyk DM, Grakoui A. Contribution of the intestinal microbiome and gut barrier to hepatic disorders. Gastroenterology. 2020; 159: 849-63.
- Tan Y, Li Y, Zhou F, Guo J, Wang T, Shi Y, et al. Administration of a mixture of triterpenoids from yeyachun and phenolic acids from danshen ameliorates carbon tetrachloride-induced liver fibrosis in mice by the regulation of intestinal flora. Journal of Pharmacological Sciences. 2020; 143: 165-75.
- Shah A, Macdonald GA, Morrison M, Holtmann G. Targeting the gut microbiome as a treatment for primary sclerosing cholangitis: a conceptional framework. The American journal of gastroenterology. 2020; 115: 814.
- Wang X, Chen L, Wang H, Cai W, Xie Q. Modulation of bile acid profile by gut microbiota in chronic hepatitis B. Journal of cellular and molecular medicine. 2020; 24: 2573-81.
- Ma XY, Zhang M, Fang G, Cheng CJ, Wang MK, Han YM, et al. Ursolic acid reduces hepatocellular apoptosis and alleviates alcohol-induced liver injury via irreversible inhibition of CASP3 in vivo. Acta Pharmacologica Sinica. 2021; 42: 1101-10.
- Chen D, Le TH, Shahidipour H, Read SA, Ahlenstiel G. The role of gut-derived microbial antigens on liver fibrosis initiation and progression. Cells. 2019; 8: 1324.
- Liu Y, Chen K, Li F, Gu Z, Liu Q, He L, et al. Probiotic Lactobacillus rhamnosus GG prevents liver fibrosis through inhibiting hepatic bile acid synthesis and enhancing bile acid excretion in mice. Hepatology. 2020; 71: 2050-66.
- Jayakumar S, Loomba R. Emerging role of the gut microbiome in the progression of nonalcoholic fatty liver disease and potential therapeutic implications. Alimentary Pharmacology & Therapeutics. 2019; 50: 144-58.
- Liu YT, Qi SL, Sun KW. Traditional Chinese medicine, liver fibrosis, intestinal flora: is there any connection?-a narrative review. Ann. Palliat. Med. 2021; 10: 4846-57.
- Gao XM. Chinese pharmacy. Beijing: China Press of Traditional Chinese Medicine. 2007; 3: 296.
- Chen LL, Verpoorte R, Yen HR, Peng WH, Cheng YC, Chao J, et al. Effects of processing adjuvants on traditional Chinese herbs. journal of food and drug analysis. 2018; 26: S96-114.
- Hamza AA, Lashin FM, Gamel M, Hassanin SO, Abdalla Y, Amin A. Hawthorn herbal preparation from Crataegus oxyacantha attenuates in vivo carbon tetrachloride-induced hepatic fibrosis via modulating oxidative stress and inflammation. Antioxidants. 2020; 9: 1173.
- Porras D, Nistal E, Martínez-Flórez S, Pisonero-Vaquero S, Olcoz JL, Jover R, et al. Protective effect of quercetin on high-fat diet-induced non-alcoholic fatty liver disease in mice is mediated by modulating intestinal microbiota imbalance and related gut-liver axis activation. Free Radical Biology and Medicine. 2017; 102: 188-202.
- Kim M, Yoo G, Randy A, Kim HS, Nho CW. Chicoric acid attenuate a nonalcoholic steatohepatitis by inhibiting key regulators of lipid metabolism, fibrosis, oxidation, and inflammation in mice with methionine and choline deficiency. Molecular nutrition & food research. 2017; 61: 1600632.
- Inamdar S, Joshi A, Malik S, Boppana R, Ghaskadbi S. Vitexin alleviates non-alcoholic fatty liver disease by activating AMPK in high fat diet fed mice. Biochemical and biophysical research communications. 2019; 519: 106-12.
- Hussain A, Cho JS, Kim JS, Lee YI. Protective effects of polyphenol enriched complex plants extract on metabolic dysfunctions associated with obesity and related nonalcoholic fatty liver diseases in high fat diet-induced c57bl/6 mice. Molecules. 2021; 26: 302.
- Li C, Chen Y, Yuan X, He L, Li X, Huang S, et al. Vitexin ameliorates chronic stress plub high fat diet-induced nonalcoholic fatty liver disease by inhibiting inflammation. European Journal of Pharmacology. 2020; 882: 173264.
- Dai X, Feng J, Chen Y, Huang S, Shi X, Liu X, Sun Y. Traditional Chinese Medicine in nonalcoholic fatty liver disease: molecular insights and therapeutic perspectives. Chinese Medicine. 2021; 16: 68.
- Liou CJ, Dai YW, Wang CL, Fang LW, Huang WC. Maslinic acid protects against obesity-induced nonalcoholic fatty liver disease in mice through regulation of the Sirt1/AMPK signaling pathway. The FASEB Journal. 2019; 33: 11791-803.
- He F, Zhang X, Wen X. Effects of Crataegolic Acid on Inflammatory Response and Oxidative Stress in Non-alcoholic Fatty Liver Disease Model Mice Induced by High-fat Diet. China Pharmacy. 2019; 2019: 901-5.
- Han X, Li W, Huang D, Yang X. Polyphenols from hawthorn peels and fleshes differently mitigate dyslipidemia, inflammation and oxidative stress in association with modulation of liver injury in high fructose diet-fed mice. Chemico-Biological Interactions. 2016; 257: 132-40.
- Hamza AA, Lashin FM, Gamel M, Hassanin SO, Abdalla Y, Amin A. Hawthorn herbal preparation from Crataegus oxyacantha attenuates in vivo carbon tetrachloride-induced hepatic fibrosis via modulating oxidative stress and inflammation. Antioxidants. 2020; 9: 1173.
- Zhang Z, Liu J, Liu Y, Shi D, He Y, Zhao P. Virtual screening of the multi-gene regulatory molecular mechanism of Si-Wu-tang against non-triple-negative breast cancer based on network pharmacology combined with experimental validation. Journal of Ethnopharmacology. 2021; 269: 113696.
- Xue X, Wu J, Ding M, Gao F, Zhou F, Xu B, et al. Si-Wu-Tang ameliorates fibrotic liver injury via modulating intestinal microbiota and bile acid homeostasis. Chinese medicine. 2021; 16: 1-20.
- Zeng S, Liu Y, Jiang C, Li B, Wen L, Feng Q. Clinical efficacy and safety of Ganshuang granules as an adjuvant treatment for chronic hepatitis B liver fibrosis: A protocol for systematic review and meta analysis. Medicine. 2020; 99: e22692.
- Shi H, Shi H, Ren F, Chen D, Chen Y, Duan Z. Naringin in Ganshuang Granule suppresses activation of hepatic stellate cells for anti-fibrosis effect by inhibition of mammalian target of rapamycin. Journal of Cellular and Molecular Medicine. 2017; 21: 500-9.
- Zhao J, Miao J, Wei X, Guo L, Li P, Lei J, et al. Traditional Chinese Medicine Ganshuang Granules Attenuate CCl4-Induced Hepatic Fibrosis by Modulating Gut Microbiota. Chemistry & Biodiversity. 2021; 18: e2100520.
- Shi H, Shi H, Ren F, Chen D, Chen Y, Duan Z. Naringin in Ganshuang Granule suppresses activation of hepatic stellate cells for anti-fibrosis effect by inhibition of mammalian target of rapamycin. Journal of Cellular and Molecular Medicine. 2017; 21: 500-9.
- Liu YM, Shi HB, Liu YR, Shi HL, Ren F, Chen Y, et al. Protective Effect of Ganshuang Granules () on Liver Cirrhosis by Suppressing Regulatory T Cells in Mouse Model. Chinese journal of integrative medicine. 2019; 25: 51-8.
- Cai FF, Wu R, Song YN, Xiong AZ, Chen XL, Yang MD, et al. Yinchenhao decoction alleviates liver fibrosis by regulating bile acid metabolism and TGF-β/Smad/ERK signalling pathway. Scientific reports. 2018; 8: 15367.
- Liu C, Sun M, Yan X, Han L, Zhang Y, Liu C, et al. Inhibition of hepatic stellate cell activation following Yinchenhao decoction administration to dimethylnitrosamine-treated rats. Hepatology Research. 2008; 38: 919-29.
- Cai FF, Bian YQ, Wu R, Sun Y, Chen XL, Yang MD, et al. Yinchenhao decoction suppresses rat liver fibrosis involved in an apoptosis regulation mechanism based on network pharmacology and transcriptomic analysis. Biomedicine & Pharmacotherapy. 2019; 114: 108863.
- Zamparelli MS, Rocco A, Compare D, Nardone G. The gut microbiota: A new potential driving force in liver cirrhosis and hepatocellular carcinoma. United European Gastroenterology Journal. 2017; 5: 944-53.
- Li H. Advances in anti hepatic fibrotic therapy with Traditional Chinese Medicine herbal formula. Journal of Ethnopharmacology. 2020; 251: 112442.
- Xu L, Xie T, Shen T, Jian S. Yinchenhao decoction for chronic hepatitis B: protocol for a systematic review and meta-analysis. Medicine. 2019; 98: e14648.
- Li N, Wang B, Wu Y, Luo X, Chen Z, Sang C, et al. Modification effects of SanWei GanJiang Powder on liver and intestinal damage through reversing bile acid homeostasis. Biomedicine & Pharmacotherapy. 2019; 116: 109044.
- Xiong T, Li H. Study on protective effect of Jiagasong Tang for liver injury animal model and cell oxidative stress model in vitro. Mol. Med. Rep. 2013; 29: 132-5.
- Li N, Wang B, Wu Y, Luo X, Chen Z, Sang C, et al. Modification effects of SanWei GanJiang Powder on liver and intestinal damage through reversing bile acid homeostasis. Biomedicine & Pharmacotherapy. 2019; 116: 109044.
- Li N, Wang B, Wu Y, Luo X, Chen Z, Sang C, et al. Modification effects of SanWei GanJiang Powder on liver and intestinal damage through reversing bile acid homeostasis. Biomedicine & Pharmacotherapy. 2019; 116: 109044.
- Lyu M, Wang YF, Fan GW, Wang XY, Xu SY, Zhu Y. Balancing herbal medicine and functional food for prevention and treatment of cardiometabolic diseases through modulating gut microbiota. Frontiers in microbiology. 2017; 8: 2146.
- Li N, Wang B, Wu Y, Luo X, Chen Z, Sang C, et al. Modification effects of SanWei GanJiang Powder on liver and intestinal damage through reversing bile acid homeostasis. Biomedicine & Pharmacotherapy. 2019; 116: 109044.
- Zhang WJ, Zhao ZY, Chang LK, Cao Y, Wang S, Kang CZ, et al. Atractylodis Rhizoma: A review of its traditional uses, phytochemistry, pharmacology, toxicology and quality control. Journal of Ethnopharmacology. 2021; 266: 113415.
- Wu QJ, Lv WL, Li JM, Zhang TT, Zhou WH, Zhang Q, et al. YinQiSanHuang Jiedu decoction for the treatment of hepatitis B-related compensated liver cirrhosis: study protocol for a multi-center randomized controlled trial. Trials. 2021; 22: 701.
- Liu YT, Qi SL, Sun KW. Traditional Chinese medicine, liver fibrosis, intestinal flora: is there any connection?-a narrative review. Ann Palliat Med. 2021; 10: 4846-57.
- Shi K, Qu L, Lin X, Xie Y, Tu J, Liu X, et al. Deep-fried atractylodis rhizoma protects against spleen deficiency-induced diarrhea through regulating intestinal inflammatory response and gut microbiota. International Journal of Molecular Sciences. 2019; 21: 124.
- Ma S, Jiang Y, Zhang B, Pang J, Xu X, Sun J, et al. Comparison of the modulatory effect on intestinal microbiota between raw and bran-fried atractylodis rhizoma in the rat model of spleen-deficiency syndrome. International Journal of Environmental Research and Public Health. 2019; 16: 3183.
- Silva LD, Rocha AMC, Rocha GA, Moura SB, Rocha MMNP, Dani R, et al. The presence of Helicobacter pylori in the liver depends on the Th1, Th17 and Treg cytokine profile of the patient. Memórias do Instituto Oswaldo Cruz. 2011; 106: 748-54.
- Yu M, Wang X, Ling F, Wang H, Zhang P, Shao S. Atractylodes lancea volatile oils attenuated helicobacter pylori NCTC11637 growth and biofilm. Microbial pathogenesis. 2019; 135: 103641.
- Inagaki N, Komatsu Y, Sasaki H, Kiyohara H, Yamada H, Ishibashi H, et al. Acidic polysaccharides from rhizomes of Atractylodes lancea as protective principle in Candida-infected mice. Planta medica. 2001; 67: 428-31.
- Wu QJ, Lv WL, Li JM, Zhang TT, Zhou WH, Zhang Q, et al. YinQiSanHuang Jiedu decoction for the treatment of hepatitis B-related compensated liver cirrhosis: study protocol for a multi-center randomized controlled trial. Trials. 2021; 22: 701.
- Zou J, Li W, Wang G, Fang S, Cai J, Wang T, et al. Hepatoprotective effects of Huangqi decoction (Astragali Radix and Glycyrrhizae Radix et Rhizoma) on cholestatic liver injury in mice: involvement of alleviating intestinal microbiota dysbiosis. Journal of Ethnopharmacology. 2021; 267: 113544.
- Du JX, Sun MY, Du GL, Li FH, Liu C, Mu YP, et al. Ingredients of Huangqi decoction slow biliary fibrosis progression by inhibiting the activation of the transforming growth factor-beta signaling pathway. BMC complementary and alternative medicine. 2012; 12: 33.
- Liu C, Wang G, Chen G, Mu Y, Zhang L, Hu X, et al. Huangqi decoction inhibits apoptosis and fibrosis, but promotes Kupffer cell activation in dimethylnitrosamine-induced rat liver fibrosis. BMC Complementary and Alternative Medicine. 2012; 12: 51.
- Song YN, Zhang GB, Lu YY, Chen QL, Yang L, Wang ZT, et al. Huangqi decoction alleviates dimethylnitrosamine-induced liver fibrosis: an analysis of bile acids metabolic mechanism. Journal of ethnopharmacology. 2016; 189: 148-56.
- Wu JS, Li YF, Li YY, Dai Y, Li WK, Zheng M, et al. Huangqi decoction alleviates alpha-naphthylisothiocyanate induced intrahepatic cholestasis by reversing disordered bile acid and glutathione homeostasis in mice. Frontiers in pharmacology. 2017; 8: 938.
- Li Y, Tang R, Leung PSC, Gershwin ME, Ma X. Bile acids and intestinal microbiota in autoimmune cholestatic liver diseases. Autoimmunity reviews. 2017; 16: 885-96.
- Liao L, Schneider KM, Galvez EJC, Frissen M, Marschall HU, Su H, et al. Intestinal dysbiosis augments liver disease progression via NLRP3 in a murine model of primary sclerosing cholangitis. Gut. 2019; 68: 1477-92.
- Tedesco D, Thapa M, Chin CY, Ge Y, Gong M, Li J, et al. Alterations in intestinal microbiota lead to production of interleukin 17 by intrahepatic γd T-cell receptor–positive cells and pathogenesis of cholestatic liver disease. Gastroenterology. 2018; 154: 2178-93.
- Zou J, Li W, Wang G, Fang S, Cai J, Wang T, et al. Hepatoprotective effects of Huangqi decoction (Astragali Radix and Glycyrrhizae Radix et Rhizoma) on cholestatic liver injury in mice: involvement of alleviating intestinal microbiota dysbiosis. Journal of Ethnopharmacology. 2021; 267: 113544.
- Xie G, Wang S, Zhang H, Zhao A, Liu J, Ma Y, et al. Poly-pharmacokinetic study of a multicomponent herbal medicine in healthy Chinese volunteers. Clinical Pharmacology & Therapeutics. 2018; 103: 692-702.
- Zhang W, Ma W, Zhang J, Song X, Sun W, Fan Y. The immunoregulatory activities of astragalus polysaccharide liposome on macrophages and dendritic cells. International journal of biological macromolecules. 2017; 105: 852-61.
- Zhao HM, Wang Y, Huang XY, Huang MF, Xu R, Yue HY, et al. Astragalus polysaccharide attenuates rat experimental colitis by inducing regulatory T cells in intestinal Peyer’s patches. World Journal of Gastroenterology. 2016; 22: 3175.
- Bhosale M, Kadthur JC, Nandi D. Roles of Salmonella enterica serovar Typhimurium encoded Peptidase N during systemic infection of Ifnγ-/- mice. Immunobiology. 2012; 217: 354-62.
- Dong N, Li X, Xue C, Wang C, Xu X, Bi C, et al. Astragalus polysaccharides attenuated inflammation and balanced the gut microflora in mice challenged with Salmonella typhimurium. International Immunopharmacology. 2019; 74: 105681.
- Li L, Yao H, Li X, Zhang Q, Wu X, Wong T, et al. Destiny of Dendrobium officinale polysaccharide after oral administration: indigestible and nonabsorbing, ends in modulating gut microbiota. Journal of agricultural and food chemistry. 2019; 67: 5968-77.
- Penetar DM, MacLean RR, McNeil JF, Lukas SE. Kudzu extract treatment does not increase the intoxicating effects of acute alcohol in human volunteers. Alcoholism: Clinical and Experimental Research. 2011; 35: 726-34.
- Li R, Xu L, Liang T, Li Y, Zhang S, Duan X. Puerarin mediates hepatoprotection against CCl4-induced hepatic fibrosis rats through attenuation of inflammation response and amelioration of metabolic function. Food and Chemical Toxicology. 2013; 52: 69-75.
- Li Y, Wang J, Asahina K. Mesothelial cells give rise to hepatic stellate cells and myofibroblasts via mesothelial–mesenchymal transition in liver injury. Proceedings of the National Academy of Sciences. 2013; 110: 2324-9.
- Xu L, Zheng N, He Q, Li R, Zhang K, Liang T. Puerarin, isolated from Pueraria lobata (Willd.), protects against hepatotoxicity via specific inhibition of the TGF-β1/Smad signaling pathway, thereby leading to anti-fibrotic effect. Phytomedicine. 2013; 20: 1172-9.
- Chen R, Liu B, Wang X, Chen K, Zhang K, Zhang L, et al. Effects of polysaccharide from Pueraria lobata on gut microbiota in mice. International Journal of Biological Macromolecules. 2020; 158: 740-9.
- Hussain A, Bose S, Wang JH, Yadav MK, Mahajan GB, Kim H. Fermentation, a feasible strategy for enhancing bioactivity of herbal medicines. Food Research International. 2016; 81: 1-6.
- Choi Y, Bose S, Shin NR, Song EJ, Nam YD, Kim H. Lactate-Fortified Puerariae radix fermented by Bifidobacterium breve improved diet-induced metabolic dysregulation via alteration of gut microbial communities. Nutrients. 2020; 12: 276.
- Wang L, Wu Y, Zhuang L, Chen X, Min H, Song S, et al. Puerarin prevents high-fat diet-induced obesity by enriching Akkermansia muciniphila in the gut microbiota of mice. PLoS One. 2019; 14: e0218490.
- Chen R, Wu P, Cai Z, Fang Y, Zhou H, Lasanajak Y, et al. Puerariae Lobatae Radix with chuanxiong Rhizoma for treatment of cerebral ischemic stroke by remodeling gut microbiota to regulate the brain–gut barriers. The Journal of nutritional biochemistry. 2019; 65: 101-14.
- Peng JH, Cui T, Sun ZL, Huang F, Chen L, Xu L, et al. Feng Q, Hu YY. Effects of puerariae radix extract on endotoxin receptors and TNF-expression induced by gut-derived endotoxin in chronic alcoholic liver injury. Evidence-Based Complementary and Alternative Medicine. 2012.